A mouse model of chemotherapy-related cognitive impairments integrating the risk factors of aging and APOE4 genotype
- PMID: 32027870
- PMCID: PMC7082850
- DOI: 10.1016/j.bbr.2020.112534
A mouse model of chemotherapy-related cognitive impairments integrating the risk factors of aging and APOE4 genotype
Abstract
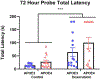
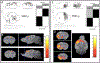
Some cancer survivors experience marked cognitive impairment, referred to as cancer-related cognitive impairment (CRCI). CRCI has been linked to the genetic factor APOE4, the strongest genetic risk factor for Alzheimer's disease (AD). We used APOE knock-in mice to test whether the relationship between APOE4 and CRCI can be demonstrated in a mouse model, to identify associations of chemotherapy with behavioural and structural correlates of cognition, and to test whether chemotherapy affects markers of AD. Twelve-month old C57BL/6 J female APOE3 (n = 30) and APOE4 (n = 31) knock-in mice were randomized to treatment with either doxorubicin (10 mg/kg) or saline. Behavioural assays at 2-21 weeks-post exposure included open field maze, elevated zero maze, pre-pulse inhibition, Barnes maze, and fear conditioning. Ex-vivo magnetic resonance imaging was used to determine regional volume differences at 31-35 weeks-post exposure, and tissue sections were analyzed for markers of AD pathogenesis. Minimal toxicities were observed in the aged mice after doxorubicin exposure. In the Barnes maze assay, APOE3 mice did not exhibit impairment in spatial learning after doxorubicin treatment, but APOE4 mice demonstrated significant impairments in both the initial identification of the escape hole and the latency to full escape at 6 weeks post-exposure. Both APOE3 and APOE4 mice treated with doxorubicin showed impairment of spatial memory. Grey matter volume in the frontal cortex decreased in APOE4 mice treated with doxorubicin vs. APOE3 mice. This study demonstrates cognitive impairments in aged APOE4 knock-in mice after doxorubicin treatment and establishes this system as a novel and powerful model of CRCI.
Keywords: Alzheimer’s disease; Apolipoprotein E; Cancer; Chemotherapy; Cognitive impairment; Mouse behaviour.
Copyright © 2020 Elsevier B.V. All rights reserved.
Figures

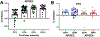

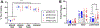
References
-
- Winocur G, Johnston I, Castel H, Chemotherapy and cognition: International cognition and cancer task force recommendations for harmonising preclinical research, Cancer Treatment Reviews 69 (2018) 72–83. - PubMed
-
- Vardy J, Tannock I, Cognitive function after chemotherapy in adults with solid tumours, Critical Reviews in Oncology/Hematology 63(3) (2007) 183–202. - PubMed
-
- Jansen CE, Miaskowski C, Dodd M, Dowling G, Kramer J, A metaanalysis of studies of the effects of cancer chemotherapy on various domains of cognitive function, Cancer 104(10) (2005) 2222–2233. - PubMed
-
- Ahles TA, Saykin AJ, McDonald BC, Li Y, Furstenberg CT, Hanscom BS, Mulrooney TJ, Schwartz GN, Kaufman PA, Longitudinal Assessment of Cognitive Changes Associated With Adjuvant Treatment for Breast Cancer: Impact of Age and Cognitive Reserve, Journal of Clinical Oncology 28(29) (2010) 4434–4440. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

