Tissue-nonspecific alkaline phosphatase is an anti-inflammatory nucleotidase
- PMID: 32028019
- PMCID: PMC7185042
- DOI: 10.1016/j.bone.2020.115262
Tissue-nonspecific alkaline phosphatase is an anti-inflammatory nucleotidase
Abstract
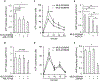
Tissue-nonspecific alkaline phosphatase (TNAP) is necessary for skeletal mineralization by its ability to hydrolyze the mineralization inhibitor inorganic pyrophosphate (PPi), which is mainly generated from extracellular ATP by ectonucleotide pyrophosphatase phosphodiesterase 1 (NPP1). Since children with TNAP deficiency develop bone metaphyseal auto-inflammations in addition to rickets, we hypothesized that TNAP also exerts anti-inflammatory effects relying on the hydrolysis of pro-inflammatory adenosine nucleotides into the anti-inflammatory adenosine. We explored this hypothesis in bone metaphyses of 7-day-old Alpl+/- mice (encoding TNAP), in mineralizing hypertrophic chondrocytes and osteoblasts, and non-mineralizing mesenchymal stem cells (MSCs) and neutrophils, which express TNAP and are present, or can be recruited in the metaphysis. Bone metaphyses of 7-day-old Alpl+/- mice had significantly increased levels of Il-1β and Il-6 and decreased levels of the anti-inflammatory Il-10 cytokine as compared with Alpl+/+ mice. In bone metaphyses, murine hypertrophic chondrocytes and osteoblasts, Alpl mRNA levels were much higher than those of the adenosine nucleotidases Npp1, Cd39 and Cd73. In hypertrophic chondrocytes, inhibition of TNAP with 25 μM of MLS-0038949 decreased the hydrolysis of AMP and ATP. However, TNAP inhibition did not significantly modulate ATP- and adenosine-associated effects in these cells. We observed that part of TNAP proteins in hypertrophic chondrocytes was sent from the cell membrane to matrix vesicles, which may explain why TNAP participated in the hydrolysis of ATP but did not significantly modulate its autocrine pro-inflammatory effects. In MSCs, TNAP did not participate in ATP hydrolysis nor in secretion of inflammatory mediators. In contrast, in neutrophils, TNAP inhibition with MLS-0038949 significantly exacerbated ATP-associated activation and secretion of IL-1β, and extended cell survival. Collectively, these results demonstrate that TNAP is a nucleotidase in both hypertrophic chondrocytes and neutrophils, and that this nucleotidase function is associated with autocrine effects on inflammation only in neutrophils.
Keywords: ATP; Hypophosphatasia; Inflammation; Nucleotidase; Tissue-nonspecific alkaline phosphatase.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest None.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

