The evolution of radiographic edema in ARDS and its association with clinical outcomes: A prospective cohort study in adult patients
- PMID: 32028223
- PMCID: PMC7136845
- DOI: 10.1016/j.jcrc.2020.01.012
The evolution of radiographic edema in ARDS and its association with clinical outcomes: A prospective cohort study in adult patients
Abstract
Purpose: To assess the longitudinal evolution of radiographic edema using chest X-rays (CXR) in patients with Acute Respiratory Distress Syndrome (ARDS) and to examine its association with prognostic biomarkers, ARDS subphenotypes and outcomes.
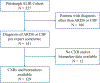
Materials and methods: We quantified radiographic edema on CXRs from patients with ARDS or cardiogenic pulmonary edema (controls) using the Radiographic Assessment of Lung Edema (RALE) score on day of intubation and up to 10 days after. We measured baseline plasma biomarkers and recorded clinical variables.
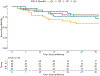
Results: The RALE score had good inter-rater agreement (r = 0.83, p < 0.0001) applied on 488 CXRs from 129 patients, with higher RALE scores in patients with ARDS (n = 108) compared to controls (n = 21, p = 0.01). Baseline RALE scores were positively correlated with levels of the receptor for end-glycation end products (RAGE) in ARDS patients (p < 0.05). Baseline RALE scores were not predictive of 30- or 90-day survival. Persistently elevated RALE scores were associated with prolonged need for mechanical ventilation (p = 0.002).
Conclusions: The RALE score is easily implementable with high inter-rater reliability. Longitudinal RALE scoring appears to be a reproducible approach to track the evolution of radiographic edema in patients with ARDS and can potentially predict prolonged need for mechanical ventilation.
Keywords: Chest X-ray; Extubation; Heterogeneity; Phenotype; Pulmonary Edema; Respiratory distress syndrome.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest Dr. Georgios Kitsios receives research funding from Karius, Inc. The other authors have no competing interests to declare.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
- K24 HL123342/HL/NHLBI NIH HHS/United States
- K08 HL144820/HL/NHLBI NIH HHS/United States
- R01 LM012087/LM/NLM NIH HHS/United States
- F32 HL137258/HL/NHLBI NIH HHS/United States
- R01 HL097376/HL/NHLBI NIH HHS/United States
- K24 HL143285/HL/NHLBI NIH HHS/United States
- R01 HL136143/HL/NHLBI NIH HHS/United States
- R01 HL142084/HL/NHLBI NIH HHS/United States
- K08 HS025455/HS/AHRQ HHS/United States
- U01 HL137159/HL/NHLBI NIH HHS/United States
- U01 HL098962/HL/NHLBI NIH HHS/United States
- K23 GM122069/GM/NIGMS NIH HHS/United States
- L30 HL143734/HL/NHLBI NIH HHS/United States
- K23 HL139987/HL/NHLBI NIH HHS/United States
- P01 HL114453/HL/NHLBI NIH HHS/United States
- F32 HL142172/HL/NHLBI NIH HHS/United States

