Crosstalk between autophagy and metabolic regulation of cancer stem cells
- PMID: 32028963
- PMCID: PMC7003352
- DOI: 10.1186/s12943-019-1126-8
Crosstalk between autophagy and metabolic regulation of cancer stem cells
Abstract
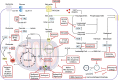
Cancer is now considered as a heterogeneous ecosystem in which tumor cells collaborate with each other and with host cells in their microenvironment. As circumstances change, the ecosystem evolves to ensure the survival and growth of the cancer cells. In this ecosystem, metabolism is not only a key player but also drives stemness. In this review, we first summarize our current understanding of how autophagy influences cancer stem cell phenotype. We emphasize metabolic pathways in cancer stem cells and discuss how autophagy-mediated regulation metabolism is involved in their maintenance and proliferation. We then provide an update on the role of metabolic reprogramming and plasticity in cancer stem cells. Finally, we discuss how metabolic pathways in cancer stem cells could be therapeutically targeted.
Keywords: Autophagy; Cancer stem cells; Lipid metabolism; Metabolic heterogeneity; Therapeutic target.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

