Arginase promotes immune evasion of Echinococcus granulosus in mice
- PMID: 32029006
- PMCID: PMC7006169
- DOI: 10.1186/s13071-020-3919-4
Arginase promotes immune evasion of Echinococcus granulosus in mice
Abstract
Background: Cystic echinococcosis is a chronic disease caused by infection with the larvae of Echinococcus granulosus. The parasite's ability to establish persistent infection is partly due to its evolving immune evasion strategies. One strategy may involve the protective effect of arginase, which impedes the control of pathogens or tumors, whereas it remains largely unknown during E. granulosus infection. Here, we analyzed whether arginase was produced in peritoneal cells and assessed its role in immunosuppression in mice infected with protoscoleces of E. granulosus.
Methods: BALB/c mice injected with protoscoleces of E. granulosus were used to evaluate the expression of arginase (ARG) in mRNA and protein levels. The profiles of ARG-1 expression in peritoneal cells and CD3ζ expression in T cells from spleens were assessed at different time points (3, 6, 9 and 12 months post-infection) by flow cytometry. In vitro, peritoneal cells were co-cultured with purified T cells in a transwell system, and the levels of CD3ζ re-expression were compared by flow cytometry. Meanwhile, the changes of L-arginine and its related metabolites in serum were tested.
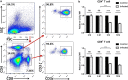
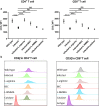
Results: Compared to the control group, the peritoneal cells from infected mice showed higher levels of ARG-1 mRNA and protein, unchanged ARG-2 and iNOS. Enhanced ARG-1 expression was present in SSClowCD11b+F4/80+, CD11b+CD11c+, CD11b+Gr-1+Ly-6C+Ly-6G-, CD11b+Gr-1+Ly-6C-Ly-6G+, CD11b+Gr-1+ and CD11b+Ly-6G+ cells. The proportion of cells and the proportion of ARG-1 expression in corresponding cells exhibited a rising trend along with the extension of infection time, except for fluctuations in SSClowCD11b+F4/80+ and CD11b+CD11c+ cells at 12 months post-infection, whereas the expression of CD3ζ chain in CD4+ and CD8+ T cells showed a descending trend. Purified T cells showed declined re-expression of CD3ζ when co-cultured with peritoneal cells from infected mice, and CD3ζ was regenerated by supplement of L-arginine or arginase inhibitor BEC, rather than NOS inhibitor L-NMMA or catalase. Meanwhile, the concentrations of L-arginine, L-citrulline and NO decreased, and those of L-ornithine and urea increased in serum post-infection.
Conclusions: Our findings demonstrated that ARG-1 expression is enhanced in multiple myeloid cells from peritoneum and promotes immune evasion of E. granulosus in mice by inhibiting the expression of T cell receptor CD3ζ chain and antagonism against iNOS.
Keywords: Arginase; Echinococcus granulosus; INOS; Immunosuppression; Peritoneum.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- Taheri F, Ochoa JB, Faghiri Z, Culotta K, Park HJ, Lan MS, et al. l-Arginine regulates the expression of the T-cell receptor zeta chain (CD3zeta) in Jurkat cells. Clin Cancer Res. 2001;7(Suppl. 3):958–965. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

