Molecular characterization of avian pathogenic Escherichia coli from broiler chickens with colibacillosis
- PMID: 32029145
- PMCID: PMC7587703
- DOI: 10.1016/j.psj.2019.10.047
Molecular characterization of avian pathogenic Escherichia coli from broiler chickens with colibacillosis
Abstract
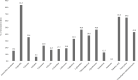
Avian pathogenic Escherichia coli (APEC) causes extensive mortality in poultry flocks, leading to extensive economic losses. The aim of this study was to investigate the phenotypic and genotypic characteristics and antimicrobial resistance of recent APEC isolates. Of the 79 APEC isolates, the most predominant serogroup was O78 (16 isolates, 20.3%), followed by O2 (7 isolates, 8.9%) and O53 (7 isolates, 8.9%). Thirty-seven (46.8%) and six (7.6%) of the isolates belonged to phylogenetic groups D and B2, respectively, and presented as virulent extraintestinal E. coli. Among 5 analyzed virulence genes, the highest frequency was observed in hlyF (74 isolates, 93.7%), followed by iutA (72 isolates, 91.9%) gene. The distribution of the iss gene was significantly different between groups A/B1 and B2/D (P < 0.05). All group B2 isolates carried all 5 virulence genes. APEC isolates showed high resistance to ampicillin (83.5%), nalidixic acid (65.8%), tetracycline (64.6%), cephalothin (46.8%), and ciprofloxacin (46.8%). The β-lactamases-encoding genes blaTEM-1 (23 isolates, 29.1%), blaCTX-M-1 (4 isolates, 5.1%), and blaCTX-M-15 (3 isolates, 3.8%); the aminoglycoside-modifying enzyme gene aac(3)-II (4 isolates, 5.1%); and the plasmid-mediated quinolone genes qnrA (10 isolates, 12.7%) and qnrS (2 isolates, 2.5%) were identified in APEC isolates. The tetA (37 isolates, 46.8%) and sul2 (20 isolates, 25.3%) were the most prevalent among tetracycline and sulfonamide resistant isolates, respectively. This study indicates that APEC isolates harbor a variety of virulence and resistance genes; such genes are often associated with plasmids that facilitate their transmission between bacteria and should be continuously monitored to track APEC transmission in poultry farms.
Keywords: antimicrobial resistance; avian pathogenic Escherichia coli; broilers; phylogenetic group.
Copyright © 2019. Published by Elsevier Inc.
Figures
References
-
- Ahmeda A.M., Shimamoto T., Shimamoto T. Molecular characterization of multidrug-resistant avian pathogenic Escherichia coli isolated from septicemic broilers. Poult. Sci. 2013;94:601–611. - PubMed
-
- Animal and Plant Quarantine Agency (APQA) Animal and Plant Quarantine Agency; Gimcheon, Republic of Korea: 2017. National Antimicrobial Resistance Monitoring Program.
-
- Barbieri N.L., de Oliveira A.L., Tejkowski T.M., Pavanelo D.B., Matter L.B., Pinheiro S.R., Vaz T.M., Nolan L.K., Logue C.M., de Brito B.G., Horn F. Molecular characterization and clonal relationships among Escherichia coli strains isolated from broiler chickens with colisepticemia. Foodborne Pathog. Dis. 2015;12:74–83. - PubMed
-
- Candrian U., Furrer B., Höfelein C., Meyer R., Jermini M., Lüthy J. Detection of Escherichia coli and identification of enterotoxigenic strains by primer-directed enzymatic amplification of specific DNA sequences. Int. J. Food Microbiol. 1991;12:339–351. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical