Ataxia-telangiectasia mutated interacts with Parkin and induces mitophagy independent of kinase activity. Evidence from mantle cell lymphoma
- PMID: 32029507
- PMCID: PMC7849759
- DOI: 10.3324/haematol.2019.234385
Ataxia-telangiectasia mutated interacts with Parkin and induces mitophagy independent of kinase activity. Evidence from mantle cell lymphoma
Abstract
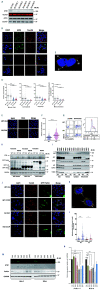
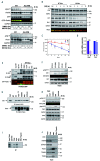
Ataxia telangiectasia mutated (ATM), a critical DNA damage sensor with protein kinase activity,is frequently altered in human cancers including mantle cell lymphoma (MCL). Loss of ATM protein is linked to accumulation of nonfunctional mitochondria and defective mitophagy, in both murine thymocytes and in A-T cells. However, the mechanistic role of ATM kinase in cancer cell mitophagy is unknown. Here, we provide evidence that FCCP-induced mitophagy in MCL and other cancer cell lines is dependent on ATM but independent of its kinase function. While Granta-519 MCL cells possess single copy and kinase dead ATM and are resistant to FCCP-induced mitophagy, both Jeko-1 and Mino cells are ATM proficient and induce mitophagy. Stable knockdown of ATM in Jeko-1 and Mino cells conferred resistance to mitophagy and was associated with reduced ATP production, oxygen consumption, and increased mROS. ATM interacts with the E3 ubiquitin ligase Parkin in a kinase-independent manner. Knockdown of ATM in HeLa cells resulted in proteasomal degradation of GFP-Parkin which was rescued by the proteasome inhibitor, MG132 suggesting that ATM-Parkin interaction is important for Parkin stability. Neither loss of ATM kinase activity in primary B cell lymphomas nor inhibition of ATM kinase in MCL, A-T and HeLa cell lines mitigated FCCP or CCCP-induced mitophagy suggesting that ATM kinase activity is dispensable for mitophagy. Malignant B-cell lymphomas without detectable ATM, Parkin, Pink1, and Parkin-Ub ser65 phosphorylation were resistant to mitophagy, providing the first molecular evidence of ATM's role in mitophagy in MCL and other B-cell lymphomas.
Figures




Similar articles
-
Lead (Pb) induced ATM-dependent mitophagy via PINK1/Parkin pathway.Toxicol Lett. 2018 Jul;291:92-100. doi: 10.1016/j.toxlet.2018.04.012. Epub 2018 Apr 13. Toxicol Lett. 2018. PMID: 29660402
-
ATM mediates spermidine-induced mitophagy via PINK1 and Parkin regulation in human fibroblasts.Sci Rep. 2016 Apr 19;6:24700. doi: 10.1038/srep24700. Sci Rep. 2016. PMID: 27089984 Free PMC article.
-
Inhibition of SKP2 Activity Impaired ATM-Mediated DNA Repair and Enhanced Sensitivity of Cisplatin-Resistant Mantle Cell Lymphoma Cells.Cancer Biother Radiopharm. 2019 Sep;34(7):451-458. doi: 10.1089/cbr.2019.2787. Epub 2019 Apr 25. Cancer Biother Radiopharm. 2019. PMID: 31025879
-
The three 'P's of mitophagy: PARKIN, PINK1, and post-translational modifications.Genes Dev. 2015 May 15;29(10):989-99. doi: 10.1101/gad.262758.115. Genes Dev. 2015. PMID: 25995186 Free PMC article. Review.
-
N-degron-mediated degradation and regulation of mitochondrial PINK1 kinase.Curr Genet. 2020 Aug;66(4):693-701. doi: 10.1007/s00294-020-01062-2. Epub 2020 Mar 10. Curr Genet. 2020. PMID: 32157382 Review.
Cited by
-
Mechanisms of MCL-1 Protein Stability Induced by MCL-1 Antagonists in B-Cell Malignancies.Clin Cancer Res. 2023 Jan 17;29(2):446-457. doi: 10.1158/1078-0432.CCR-22-2088. Clin Cancer Res. 2023. PMID: 36346691 Free PMC article.
-
Cellular functions of the protein kinase ATM and their relevance to human disease.Nat Rev Mol Cell Biol. 2021 Dec;22(12):796-814. doi: 10.1038/s41580-021-00394-2. Epub 2021 Aug 24. Nat Rev Mol Cell Biol. 2021. PMID: 34429537 Review.
-
ATM at the crossroads of reactive oxygen species and autophagy.Int J Biol Sci. 2021 Jul 22;17(12):3080-3090. doi: 10.7150/ijbs.63963. eCollection 2021. Int J Biol Sci. 2021. PMID: 34421351 Free PMC article. Review.
-
DNA damage response regulator ATR licenses PINK1-mediated mitophagy.Nucleic Acids Res. 2025 Feb 27;53(5):gkaf178. doi: 10.1093/nar/gkaf178. Nucleic Acids Res. 2025. PMID: 40105243 Free PMC article.
-
Ataxia-telangiectasia mutated plays an important role in cerebellar integrity and functionality.Neural Regen Res. 2023 Mar;18(3):497-502. doi: 10.4103/1673-5374.350194. Neural Regen Res. 2023. PMID: 36018153 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

