Fever After Influenza, Diphtheria-Tetanus-Acellular Pertussis, and Pneumococcal Vaccinations
- PMID: 32029684
- PMCID: PMC7055925
- DOI: 10.1542/peds.2019-1909
Fever After Influenza, Diphtheria-Tetanus-Acellular Pertussis, and Pneumococcal Vaccinations
Abstract
Background: Administering inactivated influenza vaccine (IIV), 13-valent pneumococcal conjugate vaccine (PCV13), and diphtheria-tetanus-acellular pertussis (DTaP) vaccine together has been associated with increased risk for febrile seizure after vaccination. We assessed the effect of administering IIV at a separate visit from PCV13 and DTaP on postvaccination fever.
Methods: In 2017-2018, children aged 12 to 16 months were randomly assigned to receive study vaccines simultaneously or sequentially. They had 2 study visits 2 weeks apart; nonstudy vaccines were permitted at visit 1. The simultaneous group received PCV13, DTaP, and quadrivalent IIV (IIV4) at visit 1 and no vaccines at visit 2. The sequential group received PCV13 and DTaP at visit 1 and IIV4 at visit 2. Participants were monitored for fever (≥38°C) and antipyretic use during the 8 days after visits.
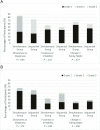
Results: There were 110 children randomly assigned to the simultaneous group and 111 children to the sequential group; 90% received ≥1 nonstudy vaccine at visit 1. Similar proportions of children experienced fever on days 1 to 2 after visits 1 and 2 combined (simultaneous [8.1%] versus sequential [9.3%]; adjusted relative risk = 0.87 [95% confidence interval 0.36-2.10]). During days 1 to 2 after visit 1, more children in the simultaneous group received antipyretics (37.4% vs 22.4%; P = .020).
Conclusions: In our study, delaying IIV4 administration by 2 weeks in children receiving DTaP and PCV13 did not reduce fever occurrence after vaccination. Reevaluating this strategy to prevent fever using an IIV4 with a different composition in a future influenza season may be considered.
Trial registration: ClinicalTrials.gov NCT03165981.
Copyright © 2020 by the American Academy of Pediatrics.
Conflict of interest statement
POTENTIAL CONFLICT OF INTEREST: Dr Klein reports potential conflicts due to support received from GlaxoSmithKline, Sanofi Pasteur, Pfizer, Merck, Protein Science (now Sanofi Pasteur), Dynavax, and MedImmune; the other authors have indicated they have no potential conflicts of interest to disclose.
Figures


References
-
- Tapiainen T, Heininger U. Fever following immunization. Expert Rev Vaccines. 2005;4(3):419–427 - PubMed
-
- Barlow WE, Davis RL, Glasser JW, et al. ; Centers for Disease Control and Prevention Vaccine Safety Datalink Working Group . The risk of seizures after receipt of whole-cell pertussis or measles, mumps, and rubella vaccine. N Engl J Med. 2001;345(9):656–661 - PubMed
-
- Tse A, Tseng HF, Greene SK, Vellozzi C, Lee GM; VSD Rapid Cycle Analysis Influenza Working Group . Signal identification and evaluation for risk of febrile seizures in children following trivalent inactivated influenza vaccine in the Vaccine Safety Datalink Project, 2010-2011. Vaccine. 2012;30(11):2024–2031 - PubMed
-
- Shimabukuro TT. Influenza end-of-season update: 2017-2018 influenza vaccine safety monitoring. In: Proceedings from the Advisory Committee on Immunization Practices meeting; June 20–21, 2018; Atlanta, GA.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

