Acetate supplementation restores chromatin accessibility and promotes tumor cell differentiation under hypoxia
- PMID: 32029721
- PMCID: PMC7005271
- DOI: 10.1038/s41419-020-2303-9
Acetate supplementation restores chromatin accessibility and promotes tumor cell differentiation under hypoxia
Abstract
Despite the fact that Otto H. Warburg discovered the Warburg effect almost one hundred years ago, why cancer cells waste most of the glucose carbon as lactate remains an enigma. Warburg proposed a connection between the Warburg effect and cell dedifferentiation. Hypoxia is a common tumor microenvironmental stress that induces the Warburg effect and blocks tumor cell differentiation. The underlying mechanism by which this occurs is poorly understood, and no effective therapeutic strategy has been developed to overcome this resistance to differentiation. Using a neuroblastoma differentiation model, we discovered that hypoxia repressed cell differentiation through reducing cellular acetyl-CoA levels, leading to reduction of global histone acetylation and chromatin accessibility. The metabolic switch triggering this global histone hypoacetylation was the induction of pyruvate dehydrogenase kinases (PDK1 and PDK3). Inhibition of PDKs using dichloroacetate (DCA) restored acetyl-CoA generation and histone acetylation under hypoxia. Knocking down PDK1 induced neuroblastoma cell differentiation, highlighting the critical role of PDK1 in cell fate control. Importantly, acetate or glycerol triacetate (GTA) supplementation restored differentiation markers expression and neuron differentiation under hypoxia. Moreover, ATAC-Seq analysis demonstrated that hypoxia treatment significantly reduced chromatin accessibility at RAR/RXR binding sites, which can be restored by acetate supplementation. In addition, hypoxia-induced histone hypermethylation by increasing 2-hydroxyglutarate (2HG) and reducing α-ketoglutarate (αKG). αKG supplementation reduced histone hypermethylation upon hypoxia, but did not restore histone acetylation or differentiation markers expression. Together, these findings suggest that diverting pyruvate flux away from acetyl-CoA generation to lactate production is the key mechanism that Warburg effect drives dedifferentiation and tumorigenesis. We propose that combining differentiation therapy with acetate/GTA supplementation might represent an effective therapy against neuroblastoma.
Conflict of interest statement
Y.L and J.Y have filed a provisional patent application: Treatment of cancer with combination of agents.
Figures

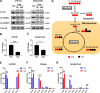
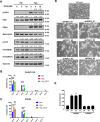
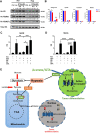
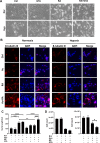
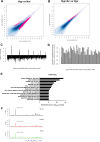
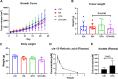

References
-
- Warburg O, Posener K, Negelein E. On the metabolism of carcinoma cells. Biochem Z. 1924;152:309–344.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

