Rapid and Precise Semi-Automatic Axon Quantification in Human Peripheral Nerves
- PMID: 32029860
- PMCID: PMC7005293
- DOI: 10.1038/s41598-020-58917-4
Rapid and Precise Semi-Automatic Axon Quantification in Human Peripheral Nerves
Erratum in
-
Author Correction: Rapid and Precise Semi-Automatic Axon Quantification in Human Peripheral Nerves.Sci Rep. 2020 Apr 17;10(1):6865. doi: 10.1038/s41598-020-63860-5. Sci Rep. 2020. PMID: 32300183 Free PMC article.
Abstract
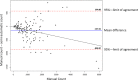
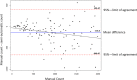
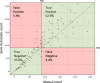
We developed a time-efficient semi-automated axon quantification method using freeware in human cranial nerve sections stained with paraphenylenediamine (PPD). It was used to analyze a total of 1238 facial and masseteric nerve biopsies. The technique was validated by comparing manual and semi-automated quantification of 129 (10.4%) randomly selected biopsies. The software-based method demonstrated a sensitivity of 94% and a specificity of 87%. Semi-automatic axon counting was significantly faster (p < 0.001) than manual counting. It took 1 hour and 47 minutes for all 129 biopsies (averaging 50 sec per biopsy, 0.04 seconds per axon). The counting process is automatic and does not need to be supervised. Manual counting took 21 hours and 6 minutes in total (average 9 minutes and 49 seconds per biopsy, 0.52 seconds per axon). Our method showed a linear correlation to the manual counts (R = 0.944 Spearman rho). Attempts have been made by several research groups to automate axonal load quantification. These methods often require specific hard- and software and are therefore only accessible to a few specialized laboratories. Our semi-automated axon quantification is precise, reliable and time-sparing using publicly available software and should be useful for an effective axon quantification in various human peripheral nerves.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Ellis, T. J., Rosen, D. & Cavanagh, J. B. Automated measurement of peripheral nerve fibres in transverse section. J. Biomed. Eng. 2 (1980). - PubMed
LinkOut - more resources
Full Text Sources

