Bone regeneration of minipig mandibular defect by adipose derived mesenchymal stem cells seeded tri-calcium phosphate- poly(D,L-lactide-co-glycolide) scaffolds
- PMID: 32029875
- PMCID: PMC7005305
- DOI: 10.1038/s41598-020-59038-8
Bone regeneration of minipig mandibular defect by adipose derived mesenchymal stem cells seeded tri-calcium phosphate- poly(D,L-lactide-co-glycolide) scaffolds
Abstract
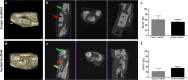
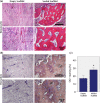
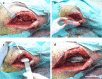
Reconstruction of bone defects represents a serious issue for orthopaedic and maxillofacial surgeons, especially in extensive bone loss. Adipose-derived mesenchymal stem cells (ADSCs) with tri-calcium phosphates (TCP) are widely used for bone regeneration facilitating the formation of bone extracellular matrix to promote reparative osteogenesis. The present study assessed the potential of cell-scaffold constructs for the regeneration of extensive mandibular bone defects in a minipig model. Sixteen skeletally mature miniature pigs were divided into two groups: Control group and scaffolds seeded with osteogenic differentiated pADSCs (n = 8/group). TCP-PLGA scaffolds with or without cells were integrated in the mandibular critical size defects and fixed by titanium osteosynthesis plates. After 12 weeks, ADSCs seeded scaffolds (n = 7) demonstrated significantly higher bone volume (34.8% ± 4.80%) than scaffolds implanted without cells (n = 6, 22.4% ± 9.85%) in the micro-CT (p < 0.05). Moreover, an increased amount of osteocalcin deposition was found in the test group in comparison to the control group (27.98 ± 2.81% vs 17.10 ± 3.57%, p < 0.001). In conclusion, ADSCs seeding on ceramic/polymer scaffolds improves bone regeneration in large mandibular defects. However, further improvement with regard to the osteogenic capacity is necessary to transfer this concept into clinical use.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Hivernaud, V. et al. Comparing “intra operative” tissue engineering strategies for the repair of craniofacial bone defects. Journal of Stomatology, Oral and Maxillofacial Surgery, 10.1016/j.jormas.2019.01.002 (2019). - PubMed
-
- Torgbo S, Sukyai P. Bacterial cellulose-based scaffold materials for bone tissue engineering. Appl. Mater. Today. 2018;11:34–49. doi: 10.1016/j.apmt.2018.01.004. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

