Energy-stress-mediated AMPK activation inhibits ferroptosis
- PMID: 32029897
- PMCID: PMC7008777
- DOI: 10.1038/s41556-020-0461-8
Energy-stress-mediated AMPK activation inhibits ferroptosis
Abstract
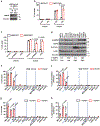
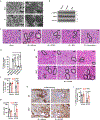
Energy stress depletes ATP and induces cell death. Here we identify an unexpected inhibitory role of energy stress on ferroptosis, a form of regulated cell death induced by iron-dependent lipid peroxidation. We found that ferroptotic cell death and lipid peroxidation can be inhibited by treatments that induce or mimic energy stress. Inactivation of AMP-activated protein kinase (AMPK), a sensor of cellular energy status, largely abolishes the protective effects of energy stress on ferroptosis in vitro and on ferroptosis-associated renal ischaemia-reperfusion injury in vivo. Cancer cells with high basal AMPK activation are resistant to ferroptosis and AMPK inactivation sensitizes these cells to ferroptosis. Functional and lipidomic analyses further link AMPK regulation of ferroptosis to AMPK-mediated phosphorylation of acetyl-CoA carboxylase and polyunsaturated fatty acid biosynthesis. Our study demonstrates that energy stress inhibits ferroptosis partly through AMPK and reveals an unexpected coupling between ferroptosis and AMPK-mediated energy-stress signalling.
Conflict of interest statement
Competing Interests
B.R.S. holds equity in and serves as a consultant to Inzen Therapeutics, and is an inventor on patents and patent applications related to ferroptosis. The other authors declare no competing financial interests.
Figures



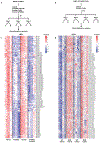
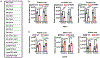
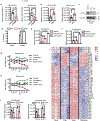

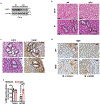





Comment in
-
Energy Stress Inhibits Ferroptotic Cell Death via AMPK Activation.Cancer Discov. 2020 Apr;10(4):485. doi: 10.1158/2159-8290.CD-RW2020-023. Epub 2020 Feb 14. Cancer Discov. 2020. PMID: 32060057
References
-
- El Mjiyad N, Caro-Maldonado A, Ramirez-Peinado S & Munoz-Pinedo C Sugar-free approaches to cancer cell killing. Oncogene 30, 253–264 (2011). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

