Mitotic slippage is determined by p31comet and the weakening of the spindle-assembly checkpoint
- PMID: 32029899
- PMCID: PMC7098889
- DOI: 10.1038/s41388-020-1187-6
Mitotic slippage is determined by p31comet and the weakening of the spindle-assembly checkpoint
Abstract
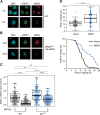
Mitotic slippage involves cells exiting mitosis without proper chromosome segregation. Although degradation of cyclin B1 during prolonged mitotic arrest is believed to trigger mitotic slippage, its upstream regulation remains obscure. Whether mitotic slippage is caused by APC/CCDC20 activity that is able to escape spindle-assembly checkpoint (SAC)-mediated inhibition, or is actively promoted by a change in SAC activity remains an outstanding issue. We found that a major culprit for mitotic slippage involves reduction of MAD2 at the kinetochores, resulting in a progressive weakening of SAC during mitotic arrest. A further level of control of the timing of mitotic slippage is through p31comet-mediated suppression of MAD2 activation. The loss of kinetochore MAD2 was dependent on APC/CCDC20, indicating a feedback control of APC/C to SAC during prolonged mitotic arrest. The gradual weakening of SAC during mitotic arrest enables APC/CCDC20 to degrade cyclin B1, cumulating in the cell exiting mitosis by mitotic slippage.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures







References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

