Clinical features of pulmonary mucormycosis in patients with different immune status
- PMID: 32030220
- PMCID: PMC6988080
- DOI: 10.21037/jtd.2019.12.53
Clinical features of pulmonary mucormycosis in patients with different immune status
Abstract
Background: Pulmonary mucormycosis (PM) is a relatively rare but often fatal and rapidly progressive disease. Most studies of PM are case reports or case series with limited numbers of patients, and focus on immunocompromised patients. We investigated the clinical manifestations, imaging features, treatment, and outcomes of patients with PM with a focus on the difference in clinical manifestations between patients with different immune status.
Methods: Clinical records, laboratory results, and computed tomography scans of 24 patients with proven or probable PM from January 2005 to December 2018 in Peking Union Medical College Hospital were retrospectively analyzed.
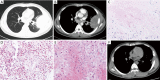
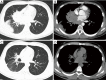
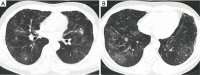
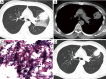
Results: Ten female and 14 male patients were included (median age, 43.5 years; range, 13-64 years). Common presenting symptoms were fever (70.8%), cough (70.8%), sputum production (54.2%), and hemoptysis (41.7%). Radiological findings included consolidation (83.3%), ground-glass opacities (58.3%), nodules (50.0%), masses (37.5%), cavities (33.3%), mediastinal lymphadenopathy (29.2%), and halo sign (12.5%); one patient had a reversed halo sign. Seven patients (29.2%) had no obvious predisposing risk factors, and 17 (70.8%) had underlying diseases including diabetes, hematological malignancy, and use of immunosuppressants. Compared with immunocompromised patients, immunocompetent patients with PM were younger {23 [13-46] vs. 48 [17-64] years, P=0.023}, comprised a higher proportion of men (100.0% vs. 41.2%, P=0.019), had a longer disease course {34 [8-47] vs. 9 [2-102] weeks, P=0.033}, had a higher eosinophil count [0.66 (0.07-2.00) ×109/L vs. 0.04 (0.00-0.23) ×109/L, P=0.001], and had a lower erythrocyte sedimentation rate {12 [1-88] vs. 74 [9-140] mm/h, P=0.032}.
Conclusions: PM can occur in heterogeneous patients with different immune status, and the clinical phenotype differs between immunocompetent and immunocompromised patients. Because of the lack of specific clinic and imaging manifestations, aggressive performance of invasive procedures to obtain histopathological and microbial evidence is crucial for a definitive diagnosis.
Keywords: Mucormycosis; fungal; lung diseases; zygomycosis.
2019 Journal of Thoracic Disease. All rights reserved.
Conflict of interest statement
Conflicts of Interest: The authors have no conflicts of interest to declare.
Figures


References
-
- Skiada A, Pagano L, Groll A, et al. Zygomycosis in Europe: analysis of 230 cases accrued by the registry of the European Confederation of Medical Mycology (ECMM) Working Group on Zygomycosis between 2005 and 2007. Clin Microbiol Infect 2011;17:1859-67. 10.1111/j.1469-0691.2010.03456.x - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
