Rationale and protocol of the Dapagliflozin And Prevention of Adverse outcomes in Chronic Kidney Disease (DAPA-CKD) randomized controlled trial
- PMID: 32030417
- PMCID: PMC7005525
- DOI: 10.1093/ndt/gfz290
Rationale and protocol of the Dapagliflozin And Prevention of Adverse outcomes in Chronic Kidney Disease (DAPA-CKD) randomized controlled trial
Abstract
Background: Recent cardiovascular outcome trials have shown that sodium-glucose co-transporter 2 (SGLT2) inhibitors slow the progression of chronic kidney disease (CKD) in patients with type 2 diabetes at high cardiovascular risk. Whether these benefits extend to CKD patients without type 2 diabetes or cardiovascular disease is unknown. The Dapagliflozin and Prevention of Adverse Outcomes in CKD (DAPA-CKD) trial (NCT03036150) will assess the effect of the SGLT2 inhibitor dapagliflozin on renal and cardiovascular events in a broad range of patients with CKD with and without diabetes.
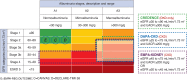
Methods: DAPA-CKD is a randomized, double-blind, placebo-controlled, trial in which ∼4300 patients with CKD Stages 2-4 and elevated urinary albumin excretion will be enrolled. The vast majority will be receiving a maximum tolerated dose of a renin-angiotensin system inhibitor at enrolment.
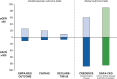
Results: After a screening assessment, eligible patients with a urinary albumin:creatinine ratio ≥200 mg/g and estimated glomerular filtration rate (eGFR) between 25 and 75 mL/min/1.73 m2 are randomly assigned to placebo or dapagliflozin 10 mg/day. Enrolment is monitored to ensure that at least 30% of patients do not have diabetes and that no more than 10% have an eGFR >60 mL/min/1.73 m2. The primary endpoint is a composite of a sustained decline in eGFR of ≥50%, end-stage renal disease, renal death or cardiovascular death. The trial will conclude when 681 primary renal events have occurred, providing 90% power to detect a 22% relative risk reduction (α level of 0.05).
Conclusion: DAPA-CKD will determine whether the SGLT2 inhibitor dapagliflozin, added to guideline-recommended therapies, safely reduces the rate of renal and cardiovascular events in patients across multiple CKD stages with and without diabetes.
Keywords: chronic kidney disease; dapagliflozin; randomized controlled clinical trial; sodium–glucose co-transporter inhibitor.
© The Author(s) 2020. Published by Oxford University Press on behalf of ERA-EDTA.
Figures


Similar articles
-
The dapagliflozin and prevention of adverse outcomes in chronic kidney disease (DAPA-CKD) trial: baseline characteristics.Nephrol Dial Transplant. 2020 Oct 1;35(10):1700-1711. doi: 10.1093/ndt/gfaa234. Nephrol Dial Transplant. 2020. PMID: 32862232 Free PMC article. Clinical Trial.
-
Effect of dapagliflozin on urinary albumin excretion in patients with chronic kidney disease with and without type 2 diabetes: a prespecified analysis from the DAPA-CKD trial.Lancet Diabetes Endocrinol. 2021 Nov;9(11):755-766. doi: 10.1016/S2213-8587(21)00243-6. Epub 2021 Oct 4. Lancet Diabetes Endocrinol. 2021. PMID: 34619106 Clinical Trial.
-
Effects of dapagliflozin on major adverse kidney and cardiovascular events in patients with diabetic and non-diabetic chronic kidney disease: a prespecified analysis from the DAPA-CKD trial.Lancet Diabetes Endocrinol. 2021 Jan;9(1):22-31. doi: 10.1016/S2213-8587(20)30369-7. Lancet Diabetes Endocrinol. 2021. PMID: 33338413 Clinical Trial.
-
Sodium-Glucose Cotransporter 2 Inhibitors in Patients with Non-Diabetic Chronic Kidney Disease.Adv Ther. 2021 May;38(5):2201-2212. doi: 10.1007/s12325-021-01735-5. Epub 2021 Apr 16. Adv Ther. 2021. PMID: 33860925 Review.
-
Barriers to early diagnosis of chronic kidney disease and use of sodium-glucose cotransporter-2 inhibitors for renal protection: A comprehensive review and call to action.Diabetes Obes Metab. 2024 Oct;26(10):4165-4177. doi: 10.1111/dom.15789. Epub 2024 Aug 14. Diabetes Obes Metab. 2024. PMID: 39140231 Review.
Cited by
-
Community Point of Care Testing in Diagnosing and Managing Chronic Kidney Disease.Diagnostics (Basel). 2024 Jul 17;14(14):1542. doi: 10.3390/diagnostics14141542. Diagnostics (Basel). 2024. PMID: 39061680 Free PMC article. Review.
-
Extrapolated longer-term effects of the DAPA-CKD trial: a modelling analysis.Nephrol Dial Transplant. 2023 May 4;38(5):1260-1270. doi: 10.1093/ndt/gfac280. Nephrol Dial Transplant. 2023. PMID: 36301617 Free PMC article. Clinical Trial.
-
Dapagliflozin Ameliorates Lipopolysaccharide Related Acute Kidney Injury in Mice with Streptozotocin-induced Diabetes Mellitus.Int J Med Sci. 2022 Apr 4;19(4):729-739. doi: 10.7150/ijms.69031. eCollection 2022. Int J Med Sci. 2022. PMID: 35582427 Free PMC article.
-
Empagliflozin and colchicine in patients with reduced left ventricular ejection fraction following ST-elevation myocardial infarction: a randomized, double-blinded, three-arm parallel-group, controlled trial.Eur J Clin Pharmacol. 2024 Jan;80(1):93-104. doi: 10.1007/s00228-023-03582-5. Epub 2023 Oct 28. Eur J Clin Pharmacol. 2024. PMID: 37897527 Clinical Trial.
-
Evaluation of the Pharmacokinetics and Exposure-Response Relationship of Dapagliflozin in Patients without Diabetes and with Chronic Kidney Disease.Clin Pharmacokinet. 2021 Apr;60(4):517-525. doi: 10.1007/s40262-020-00956-1. Epub 2021 Feb 15. Clin Pharmacokinet. 2021. PMID: 33587286 Free PMC article. Clinical Trial.
References
-
- Heerspink HJ, Perkins BA, Fitchett D. et al. Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes: cardiovascular and kidney effects, potential mechanisms and clinical applications. Circulation 2016; 134: 752–772 - PubMed
-
- Zinman B, Wanner C, Lachin JM. et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373: 2117–2128 - PubMed
-
- Neal B, Perkovic V, Mahaffey KW. et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017; 377: 644–657 - PubMed
-
- Wiviott SD, Raz I, Bonaca MP. et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019; 380: 347–357 - PubMed
-
- Perkovic V, Jardine MJ, Neal B. et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 2019; 380: 2295–2306 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

