Safety of drug use in patients with a primary mitochondrial disease: An international Delphi-based consensus
- PMID: 32030781
- PMCID: PMC7383489
- DOI: 10.1002/jimd.12196
Safety of drug use in patients with a primary mitochondrial disease: An international Delphi-based consensus
Abstract
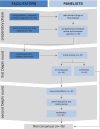
Clinical guidance is often sought when prescribing drugs for patients with primary mitochondrial disease. Theoretical considerations concerning drug safety in patients with mitochondrial disease may lead to unnecessary withholding of a drug in a situation of clinical need. The aim of this study was to develop consensus on safe medication use in patients with a primary mitochondrial disease. A panel of 16 experts in mitochondrial medicine, pharmacology, and basic science from six different countries was established. A modified Delphi technique was used to allow the panellists to consider draft recommendations anonymously in two Delphi rounds with predetermined levels of agreement. This process was supported by a review of the available literature and a consensus conference that included the panellists and representatives of patient advocacy groups. A high level of consensus was reached regarding the safety of all 46 reviewed drugs, with the knowledge that the risk of adverse events is influenced both by individual patient risk factors and choice of drug or drug class. This paper details the consensus guidelines of an expert panel and provides an important update of previously established guidelines in safe medication use in patients with primary mitochondrial disease. Specific drugs, drug groups, and clinical or genetic conditions are described separately as they require special attention. It is important to emphasise that consensus-based information is useful to provide guidance, but that decisions related to drug prescribing should always be tailored to the specific needs and risks of each individual patient. We aim to present what is current knowledge and plan to update this regularly both to include new drugs and to review those currently included.
Keywords: drugs; in vitro studies; in vivo studies; mitochondrial diseases; mitochondrial toxicity; safety.
© 2020 The Authors. Journal of Inherited Metabolic Disease published by John Wiley & Sons Ltd on behalf of SSIEM.
Conflict of interest statement
M. A. reports grants from Virginia Tech Interdisciplinary Graduate Education Program, outside the submitted work.
D. B. reports grants from National Institutes of Health, grants and personal fees from Stealth BioTherapeutics, grants from vTv Therapeutics, grants from Catabasis Pharmaceuticals, grants from United States Dept of Agriculture, outside the submitted work.
A. K. reports that she is the current President of the Mitochondrial Medicine Society.
R. M. reports personal fees from Eisai, outside the submitted work.
S. R. reports personal fees from BioMedical, personal fees from NeuroVive, personal fees from Partners 4 Access, outside the submitted work, and that she is an Editor of the Journal of Inherited Metabolic Disease.
T. S. reports grants from Princes Beatrix Muscle Foundation (Prinses Beatrix Spierfonds), grants from European Molecular Biology Organization, during the conduct of the study.
L. B., G. G., N. K., C. L., M. M., R. M., Y. S. N., M. O., R. P., F. R., K. V., and M. C. De V. declare that they have no conflict of interest.
Figures
References
-
- Rahman J, Rahman S. Mitochondrial medicine in the omics era. Lancet. 2018;391:2560‐2574. - PubMed
-
- Gorman GS, Chinnery PF, DiMauro S, et al. Mitochondrial diseases. Nat Rev Dis Primers. 2016;2:16080. - PubMed
-
- Hargreaves IP, Al Shahrani M, Wainwright L, et al. Drug‐induced mitochondrial toxicity. Drug Saf. 2016;39:661‐674. - PubMed
-
- Chan K, Truong D, Shangari N, O'Brien PJ. Drug‐induced mitochondrial toxicity. Expert Opin Drug Metab Toxicol. 2005;1:655‐669. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical