Autocatalytic Carbonyl Arylation through In Situ Release of Aryl Nucleophiles from N-Aryl-N'-Silyldiazenes
- PMID: 32030857
- PMCID: PMC7383908
- DOI: 10.1002/anie.201916004
Autocatalytic Carbonyl Arylation through In Situ Release of Aryl Nucleophiles from N-Aryl-N'-Silyldiazenes
Abstract
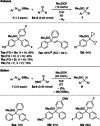
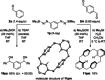
A method for the catalytic generation of functionalized aryl alkali metals is reported. These highly reactive intermediates are liberated from silyl-protected aryl-substituted diazenes by the action of Lewis basic alkali metal silanolates, resulting in desilylation and loss of N2 . Catalytic quantities of these Lewis bases initiate the transfer of the aryl nucleophile from the diazene to carbonyl and carboxyl compounds with superb functional-group tolerance. The aryl alkali metal can be decorated with electrophilic substituents such as methoxycarbonyl or cyano as well as halogen groups. The synthesis of a previously unknown cyclophane-like [4]arene macrocycle from a 1,3-bisdiazene combined with a 1,4-dialdehyde underlines the potential of the approach.
Keywords: Lewis bases; autocatalysis; chemoselectivity; nucleophilic addition; silicon.
© 2020 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Organometallics in Synthesis: Third Manual (Ed.: M. Schlosser), Wiley, Hoboken, 2013.
-
- None
-
- Benischke A. D., Ellwart M., Becker M. R., Knochel P., Synthesis 2016, 48, 1101–1107;
-
- Dagousset G., François C., León T., Blanc R., Sansiaume-Dagousset E., Knochel P., Synthesis 2014, 46, 3133–3171;
-
- Knochel P., Dohle W., Gommermann N., Kneisel F. F., Kopp F., Korn T., Sapountzis I., Vu V. A., Angew. Chem. Int. Ed. 2003, 42, 4302–4320; - PubMed
- Angew. Chem. 2003, 115, 4438–4456.
Grants and funding
LinkOut - more resources
Full Text Sources

