V(D)J recombination, somatic hypermutation and class switch recombination of immunoglobulins: mechanism and regulation
- PMID: 32031242
- PMCID: PMC7341547
- DOI: 10.1111/imm.13176
V(D)J recombination, somatic hypermutation and class switch recombination of immunoglobulins: mechanism and regulation
Abstract
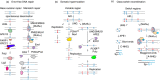
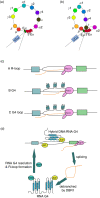
Immunoglobulins emerging from B lymphocytes and capable of recognizing almost all kinds of antigens owing to the extreme diversity of their antigen-binding portions, known as variable (V) regions, play an important role in immune responses. The exons encoding the V regions are known as V (variable), D (diversity), or J (joining) genes. V, D, J segments exist as multiple copy arrays on the chromosome. The recombination of the V(D)J gene is the key mechanism to produce antibody diversity. The recombinational process, including randomly choosing a pair of V, D, J segments, introducing double-strand breaks adjacent to each segment, deleting (or inverting in some cases) the intervening DNA and ligating the segments together, is defined as V(D)J recombination, which contributes to surprising immunoglobulin diversity in vertebrate immune systems. To enhance both the ability of immunoglobulins to recognize and bind to foreign antigens and the effector capacities of the expressed antibodies, naive B cells will undergo class switching recombination (CSR) and somatic hypermutation (SHM). However, the genetics mechanisms of V(D)J recombination, CSR and SHM are not clear. In this review, we summarize the major progress in mechanism studies of immunoglobulin V(D)J gene recombination and CSR as well as SHM, and their regulatory mechanisms.
Keywords: B cell; V(D)J recombination; class switch recombination; mechanism; somatic hypermutation.
© 2020 John Wiley & Sons Ltd.
Figures


References
-
- Boyd SD, Joshi SA. High‐throughput DNA sequencing analysis of antibody repertoires. Microbiol Spectr 2014; 2:345–62. - PubMed
Publication types
MeSH terms
Grants and funding
- 91642206/National Natural Science Foundation's Major Research Programme
- XDA16010500/The Strategic Priority Research Programme of the Chinese Academy of Sciences, China
- 81320108020/Major International Cooperation Projects of the National Natural Science Foundation
- 31625016/The National Natural Science Fund for Distinguished Young Scholars
- BMU2018JDJS010/The Research Institute Fund of the NHC Key Laboratory of Medical Immunology, Peking University
- 2017PT31037/The Non-profifit Central Research Institute Fund of the Chinese Academy of Medical Sciences
- JCYJ20170413141047772/Shenzhen Science and Technology Programme
- JCYJ20180507181659781/Shenzhen Science and Technology Programme
- QYZDY-SSW-SMC027/CAS Key Research Projects of Frontier Science
- 2018133/The CAS Youth Innovation Promotion Association
- 2016097/The CAS Youth Innovation Promotion Association
- 2017SHZDZX01/The Shanghai Municipal Science and Technology Major Project
LinkOut - more resources
Full Text Sources

