Vitamin-D3 (α-1, 25(OH) 2D3) Protects Retinal Pigment Epithelium From Hyperoxic Insults
- PMID: 32031576
- PMCID: PMC7325624
- DOI: 10.1167/iovs.61.2.4
Vitamin-D3 (α-1, 25(OH) 2D3) Protects Retinal Pigment Epithelium From Hyperoxic Insults
Abstract
Purpose: Oxidative stress affects the retinal pigment epithelium (RPE) leading to development of vascular eye diseases. Cholecalciferol (VIT-D) is a known modulator of oxidative stress and angiogenesis. This in vitro study was carried out to evaluate the protective role of VIT-D on RPE cells incubated under hyperoxic conditions.
Methods: Cadaver primary RPE (PRPE) cells were cultured in hyperoxia (40% O2) with or without VIT-D (α-1, 25(OH) 2D3). The functional and physiological effects of PRPE cells with VIT-D treatment were analyzed using molecular and biochemical tools.
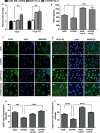
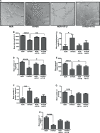
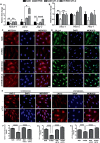
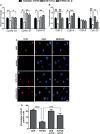
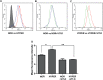
Results: Vascular signaling modulators, such as vascular endothelial growth factor (VEGF) and Notch, were reduced in hyperoxic conditions but significantly upregulated in the presence of VIT-D. Additionally, PRPE conditioned medium with VIT-D induced the tubulogenesis in primary human umbilical vein endothelial cells (HUVEC) cells. VIT-D supplementation restored phagocytosis and transmembrane potential in PRPE cells cultured under hyperoxia.
Conclusions: VIT-D protects RPE cells and promotes angiogenesis under hyperoxic insult. These findings may give impetus to the potential of VIT-D as a therapeutic agent in hyperoxia induced retinal vascular diseases.
Conflict of interest statement
Disclosure:
Figures


References
-
- Roberts PA, Gaffney EA, Whiteley JP, et al. .. Predictive mathematical models for the spread and treatment of hyperoxia-induced photoreceptor degeneration in retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2018; 59: 1238–1249. - PubMed
-
- Shen CL, Samathanam C, Graham S, et al. .. Green tea polyphenols and 1-alpha-OH-vitamin D(3) attenuate chronic inflammation-induced myocardial fibrosis in female rats. J Med Food. 2012; 15: 269–277. - PubMed
-
- Iseki K, Tatsuta M, Uehara H, et al. .. Inhibition of angiogenesis as a mechanism for inhibition by 1alpha-hydroxyvitamin D3 and 1,25-dihydroxyvitamin D3 of colon carcinogenesis induced by azoxymethane in Wistar rats. Int J Cancer. 1999; 81: 730–733. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

