Ixekizumab treatment of biologic-naïve patients with active psoriatic arthritis: 3-year results from a phase III clinical trial (SPIRIT-P1)
- PMID: 32031665
- PMCID: PMC7516094
- DOI: 10.1093/rheumatology/kez684
Ixekizumab treatment of biologic-naïve patients with active psoriatic arthritis: 3-year results from a phase III clinical trial (SPIRIT-P1)
Abstract
Objective: The aim was to assess the safety and efficacy of up to 156 weeks of ixekizumab (an IL-17A antagonist) treatment in PsA patients.
Methods: In a phase III study, patients naïve to biologic treatment were randomized to placebo, adalimumab 40 mg every 2 weeks (ADA; active reference) or ixekizumab 80 mg every 2 weeks (IXEQ2W) or every 4 weeks (IXEQ4W) after an initial dose of 160 mg. At week 24 (week 16 for inadequate responders), ADA (after 8-week washout) and placebo patients were re-randomized to IXEQ2W or IXEQ4W. Outcomes were evaluated using a modified non-responder imputation [linear extrapolation for radiographic progression (modified total Sharp score = 0)] during extended treatment until week 156.
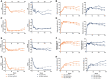
Results: Of 417 patients, 381 entered the extension, and 243 of 381 (63.8%) completed the 156-week study. Incidence rates of treatment-emergent and serious adverse events, respectively, were 38.0 and 5.2 with IXEQ2W (n = 189) and 38.1 and 8.0 with IXEQ4W (n = 197). One death occurred (IXEQ4W). With IXEQ2W and IXEQ4W, respectively, the response rates persisted to week 156 as measured by the ACR response ≥20% (62.5 and 69.8%), ≥50% (56.1 and 51.8%) and ≥70% (43.8 and 33.4%), psoriasis area and severity index (PASI) 75 (69.1 and 63.5%), PASI 90 (64.5 and 51.2%) and PASI 100 (60.5 and 43.6%). Inhibition of radiographic progression also persisted to week 156 in 61% of IXEQ2W and 71% of IXEQ4W patients.
Conclusion: In this 156-week study of ixekizumab, the safety profile remained consistent with previous reports, and improvements in signs and symptoms of PsA were observed, including persistent low rates of radiographic progression.
Trial registration: ClinicalTrials.gov, http://clinicaltrials.gov, NCT01695239, EudraCT 2011-002326-49.
Keywords: DMARDs (biologic); ixekizumab; psoriasis; psoriatic arthritis; spondyloarthritis.
© The Author(s) 2020. Published by Oxford University Press on behalf of the British Society for Rheumatology.
Figures


References
-
- Ritchlin CT, Colbert RA, Gladman DD.. Psoriatic arthritis. N Engl J Med 2017;376:957–70. Erratum in: N Engl J Med 2017;376:2097. - PubMed
-
- Sakkas LI, Bogdanos DP.. Are psoriasis and psoriatic arthritis the same disease? The IL-23/IL-17 axis data. Autoimmun Rev 2017;16:10–5. - PubMed
-
- Mease PJ, van der Heijde D, Ritchlin CT. et al. Ixekizumab, an interleukin-17A specific monoclonal antibody, for the treatment of biologic-naive patients with active psoriatic arthritis: results from the 24-week randomised, double-blind, placebo-controlled and active (adalimumab)-controlled period of the phase III trial SPIRIT-P1. Ann Rheum Dis 2017;76:79–87. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

