An overview of precision oncology basket and umbrella trials for clinicians
- PMID: 32031692
- PMCID: PMC7187272
- DOI: 10.3322/caac.21600
An overview of precision oncology basket and umbrella trials for clinicians
Abstract
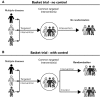
With advancements in biomarkers and momentum in precision medicine, biomarker-guided trials such as basket trials and umbrella trials have been developed under the master protocol framework. A master protocol refers to a single, overarching design developed to evaluate multiple hypotheses with the general goal of improving the efficiency of trial evaluation. One type of master protocol is the basket trial, in which a targeted therapy is evaluated for multiple diseases that share common molecular alterations or risk factors that may help predict whether the patients will respond to the given therapy. Another variant of a master protocol is the umbrella trial, in which multiple targeted therapies are evaluated for a single disease that is stratified into multiple subgroups based on different molecular or other predictive risk factors. Both designs follow the core principle of precision medicine-to tailor intervention strategies based on the patient's risk factor(s) that can help predict whether they will respond to a specific treatment. There have been increasing numbers of basket and umbrella trials, but they are still poorly understood. This article reviews common characteristics of basket and umbrella trials, key trials and recent US Food and Drug Administration approvals for precision oncology, and important considerations for clinical readers when critically evaluating future publications on basket trials and umbrella trials and for researchers when designing these clinical trials.
Keywords: basket trials; master protocols; precision medicine; precision oncology; umbrella trials.
© 2020 The Authors. CA: A Cancer Journal for Clinicians published by Wiley Periodicals, Inc. on behalf of American Cancer Society.
Figures

References
-
- Abrams J, Conley B, Mooney M, et al. National Cancer Institute's precision medicine initiatives for the new national clinical trials network. Am Soc Clin Oncol Educ Book. 2014;34:71‐76. - PubMed
-
- Ashley EA. The precision medicine initiative: a new national effort. JAMA. 2015;313:2119‐2120. - PubMed
-
- Ashley EA. Towards precision medicine. Nat Rev Genet. 2016;17:507‐522. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

