Biodistribution and toxicity of epitope-functionalized dextran iron oxide nanoparticles in a pregnant murine model
- PMID: 32031743
- PMCID: PMC8259460
- DOI: 10.1002/jbm.a.36893
Biodistribution and toxicity of epitope-functionalized dextran iron oxide nanoparticles in a pregnant murine model
Abstract
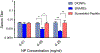
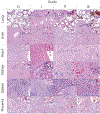
In pursuit of a preventive therapeutic for maternal autoantibody-related (MAR) autism, we assessed the toxicity, biodistribution, and clearance of a MAR specific peptide-functionalized dextran iron oxide nanoparticle system in pregnant murine dams. We previously synthesized ~15 nm citrate-coated dextran iron oxide nanoparticles (DIONPs), surface-modified with polyethylene glycol and MAR peptides to produce systems for nanoparticle-based autoantibody reception and entrapments (SNAREs). First, we investigated their immunogenicity and MAR lactate dehydrogenase B antibody uptake in murine serum in vitro. To assess biodistribution and toxicity, as well as systemic effects, we performed in vivo clinical and post mortem pathological evaluations. We observed minimal production of inflammatory cytokines-interleukin 10 (IL-10) and IL-12 following in vitro exposure of macrophages to SNAREs. We established the maximum tolerated dose of SNAREs to be 150 mg/kg at which deposition of iron was evident in the liver and lungs by histology and magnetic resonance imaging but no concurrent evidence of liver toxicity or lung infarction was detected. Further, SNAREs exhibited slower clearance from the maternal blood in pregnant dams compared to DIONPs based on serum total iron concentration. These findings demonstrated that the SNAREs have a prolonged presence in the blood and are safe for use in pregnant mice as evidenced by no associated organ damage, failure, inflammation, and fetal mortality. Determination of the MTD dose sets the basis for future studies investigating the efficacy of our nanoparticle formulation in a MAR autism mouse model.
Keywords: MAR autism; clearance; distribution; histopathology; nanoformulation; peptide-functionalized.
© 2020 Wiley Periodicals, Inc.
Conflict of interest statement
CONFLICT OF INTEREST
All other authors have no conflict of interest to declare.
Figures

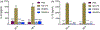




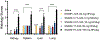




References
-
- Ali Noori KP, Modaresi M, Messripour M, Yousefi MH, & Amiri GR (2011). Effect of magnetic iron oxide nanoparticles on pregnancy and testicular development of mice. African Journal of Biotechnology, 10(7), 1221–1227.
-
- Athie-Morales V, Smits HH, Cantrell DA, & Hilkens CM (2004). Sustained IL-12 signaling is required for Th1 development. Journal of Immunology, 172(1), 61–69. - PubMed

