Role of the interferons in CD64 and CD169 expressions in whole blood: Relevance in the balance between viral- or bacterial-oriented immune responses
- PMID: 32031762
- PMCID: PMC7016842
- DOI: 10.1002/iid3.289
Role of the interferons in CD64 and CD169 expressions in whole blood: Relevance in the balance between viral- or bacterial-oriented immune responses
Abstract
Introduction: CD64 expression increases on neutrophils during bacterial infections. Recently an increase in CD169 expression has been discovered on monocytes during viral infections. Generally, interferons α (IFNsα) and IFNsγ are key drivers of the infectious host immune response. The purpose of this study was to explore if a link exists between these IFNs and both biomarkers.
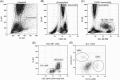
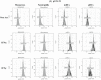
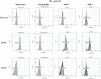
Methods: Whole blood samples from healthy volunteers were stimulated with either IFNs, interleukins, or infectious extracts, to mimic an infectious state. Expressions of CD64 and CD169 were assessed in these samples by multiple flow cytometry methods, over precise kinetics.
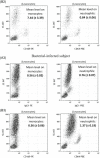
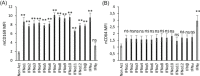
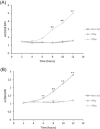
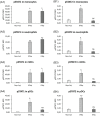
Results: The expression of CD64 was statistically higher in samples stimulated with IFNγ, and CD169 in those stimulated with IFNα (and all other type I IFNs). Surface expressions are directly induced by their respective IFNs via Janus kinase/signal transducer and activator of transduction pathways within 6 to 8 hours of incubation. Mixing both types of IFNs seemed to indicate that they partially inhibit each other.
Conclusions: The induction of CD169 on monocytes and CD164 on neutrophils by type I and type II IFNs confirms the relevance of these markers for assessing between a viral- vs bacterial-oriented immune response.
Keywords: CD169; CD64; flow cytometry; interferons; pathways.
© 2020 The Authors. Immunity, Inflammation and Disease published by John Wiley & Sons Ltd.
Conflict of interest statement
Pénélope Bourgoin, Géraldine Biéchelé, Inès Ait Belkacem, and Fabrice Malergue are Beckman Coulter employees. Beckman Coulter and the Beckman Coulter product and service marks mentioned herein are trademarks or registered trademarks of Beckman Coulter, Inc, in the United States and other countries. All other trademarks are the property of their respective owners.
Figures








References
-
- Murphy K, Weaver C. Janeway's Immunobiology. 9th ed. New York NY: Garland Science/Taylor & Francis Group, LLC; 2017.
-
- Luis Muñoz‐Carrillo J, Francisco Contreras‐Cordero J, Gutiérrez‐Coronado O, Trinidad Villalobos‐Gutiérrez P, Guillermo Ramos‐Gracia L, Elizabeth Hernández‐Reyes V. Cytokine profiling plays a crucial role in activating immune system to clear infectious pathogens In: Tyagi R K, Bisen P S, eds. Immune Response Activation and Immunomodulation. Mexico: IntechOpen; 2019:1‐30.
-
- Engelmann I, Dubos F, Lobert P‐E, et al. Diagnosis of viral infections using myxovirus resistance protein A (MxA). Pediatrics. 2015;135(4):e985‐e993. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

