EV-101: A Phase I Study of Single-Agent Enfortumab Vedotin in Patients With Nectin-4-Positive Solid Tumors, Including Metastatic Urothelial Carcinoma
- PMID: 32031899
- PMCID: PMC7106979
- DOI: 10.1200/JCO.19.02044
EV-101: A Phase I Study of Single-Agent Enfortumab Vedotin in Patients With Nectin-4-Positive Solid Tumors, Including Metastatic Urothelial Carcinoma
Erratum in
-
Erratum.J Clin Oncol. 2022 May 20;40(15):1711. doi: 10.1200/JCO.22.00785. J Clin Oncol. 2022. PMID: 35580363 Free PMC article. No abstract available.
Abstract
Purpose: To assess the safety/tolerability and antitumor activity of enfortumab vedotin (EV), a novel investigational antibody-drug conjugate that delivers the microtubule-disrupting agent, monomethyl auristatin E, to cells that express Nectin-4.
Methods: EV-101 is a phase I dose escalation/expansion study that enrolled patients with Nectin-4-expressing solid tumors (eg, metastatic urothelial carcinoma [mUC]) who progressed on ≥ 1 prior chemotherapy regimen and/or programmed death-1 receptor/programmed death ligand-1 [PD-(L)1] inhibitor, including a cohort of patients with mUC who received prior anti-PD-(L)1 therapy. Patients received escalating doses of EV up to 1.25 mg/kg on days 1, 8, and 15 of every 28-day cycle. Primary objectives were evaluation of safety/tolerability and pharmacokinetics; antitumor activity was a secondary objective.
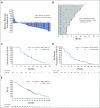
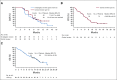
Results: Enrolled patients with mUC (n = 155) were heavily pretreated, with 96% having prior platinum-based chemotherapy and 29% receiving ≥ 3 lines of prior treatment. Maximum tolerated dose of EV was not established; however, the recommended phase II dose was identified as 1.25 mg/kg. Rash, peripheral neuropathy, fatigue, alopecia, and nausea were the most common treatment-related adverse events (TRAEs); the most common TRAEs were grade 1-2 in severity. Among the 112 patients with mUC treated with single-agent EV 1.25 mg/kg, the investigator-assessed confirmed objective response rate (ORR) was 43%, and duration of response was 7.4 months. Median overall survival (OS) was 12.3 months, and the OS rate at 1 year was 51.8%. Similar ORR and estimated median OS were observed in patients ≥ 75 years of age with and without prior anti-PD-(L)1 treatment, liver metastases, or upper-tract disease.
Conclusion: Single-agent EV was generally well tolerated and provided clinically meaningful and durable responses in patients with mUC; survival data are encouraging. A pivotal phase II and a confirmatory phase III study are ongoing.
Figures
References
-
- Challita-Eid PM, Satpayev D, Yang P, et al. Enfortumab vedotin antibody-drug conjugate targeting Nectin-4 is a highly potent therapeutic agent in multiple preclinical cancer models. Cancer Res. 2016;76:3003–3013. - PubMed
-
- Rikitake Y, Mandai K, Takai Y. The role of nectins in different types of cell-cell adhesion. J Cell Sci. 2012;125:3713–3722. - PubMed
-
- Uhlén M, Fagerberg L, Hallström BM, et al. Proteomics. Tissue-based map of the human proteome. Science. 2015;347:1260419. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

