Nusinersen treatment and cerebrospinal fluid neurofilaments: An explorative study on Spinal Muscular Atrophy type 3 patients
- PMID: 32032473
- PMCID: PMC7077557
- DOI: 10.1111/jcmm.14939
Nusinersen treatment and cerebrospinal fluid neurofilaments: An explorative study on Spinal Muscular Atrophy type 3 patients
Abstract
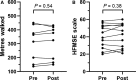
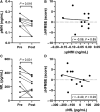
The antisense oligonucleotide Nusinersen has been recently licensed to treat spinal muscular atrophy (SMA). Since SMA type 3 is characterized by variable phenotype and milder progression, biomarkers of early treatment response are urgently needed. We investigated the cerebrospinal fluid (CSF) concentration of neurofilaments in SMA type 3 patients treated with Nusinersen as a potential biomarker of treatment efficacy. The concentration of phosphorylated neurofilaments heavy chain (pNfH) and light chain (NfL) in the CSF of SMA type 3 patients was evaluated before and after six months since the first Nusinersen administration, performed with commercially available enzyme-linked immunosorbent assay (ELISA) kits. Clinical evaluation of SMA patients was performed with standardized motor function scales. Baseline neurofilament levels in patients were comparable to controls, but significantly decreased after six months of treatment, while motor functions were only marginally ameliorated. No significant correlation was observed between the change in motor functions and that of neurofilaments over time. The reduction of neurofilament levels suggests a possible early biochemical effect of treatment on axonal degeneration, which may precede changes in motor performance. Our study mandates further investigations to assess neurofilaments as a marker of treatment response.
Keywords: Nusinersen; neurofilaments; pharmacodynamics biomarker; spinal muscular atrophy.
© 2020 The Authors. Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors confirm that there are no conflicts of interest.
Figures

References
-
- Wang CH, Finkel RS, Bertini ES, et al. Consensus statement for standard of care in spinal muscular atrophy. J Child Neurol. 2007;22:1027‐1049. - PubMed
-
- Calucho M, Bernal S, Alías L, et al. Correlation between SMA type and SMN2 copy number revisited: An analysis of 625 unrelated Spanish patients and a compilation of 2834 reported cases. Neuromuscul Disord NMD. 2018;28:208‐215. - PubMed
-
- Wirth B, Garbes L, Riessland M. How genetic modifiers influence the phenotype of spinal muscular atrophy and suggest future therapeutic approaches. Curr Opin Genet Dev. 2013;23:330‐338. - PubMed
-
- Finkel RS, Mercuri E, Darras BT, et al. Nusinersen versus Sham Control in Infantile‐Onset Spinal Muscular Atrophy. N Engl J Med. 2017;377:1723‐1732. - PubMed

