Optimal identification of human conventional and nonconventional (CRTH2-IL7Rα-) ILC2s using additional surface markers
- PMID: 32032632
- PMCID: PMC7398840
- DOI: 10.1016/j.jaci.2020.01.038
Optimal identification of human conventional and nonconventional (CRTH2-IL7Rα-) ILC2s using additional surface markers
Abstract
Background: Human type 2 innate lymphoid cells (ILC2s) are identified by coupled detection of CRTH2 and IL7Rα on lineage negative (Lin-) cells. Type 2 cytokine production by CRTH2-IL7Rα- innate lymphoid cells (ILCs) is unknown.
Objective: We sought to identify CRTH2-IL7Rα- type 2 cytokine-producing ILCs and their disease relevance.
Methods: We studied human blood and lung ILCs from asthmatic and control subjects by flow cytometry, ELISA, RNA sequencing, quantitative PCR, adoptive transfer to mice, and measurement of airway hyperreactivity by Flexivent.
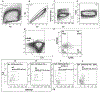
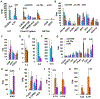
Results: We found that IL-5 and IL-13 were expressed not only by CRTH2+ but also by CRTH2-IL7Rα+ and CRTH2-IL7Rα- (double-negative [DN]) human blood and lung cells. All 3 ILC populations expressed type 2 genes and induced airway hyperreactivity when adoptively transferred to mice. The frequency of type 2 cytokine-positive IL7Rα and DN ILCs were similar to that of CRTH2 ILCs in the blood and lung. Their frequency was higher in asthmatic patients than in disease controls. Transcriptomic analysis of CRTH2, IL7Rα, and DN ILCs confirmed the expression of mRNA for type 2 transcription factors in all 3 populations. Unexpectedly, the mRNA for GATA3 and IL-5 correlated better with mRNA for CD30, TNFR2, ICOS, CCR4, and CD200R1 than for CRTH2. By using a combination of these surface markers, especially CD30/TNFR2, we identified a previously unrecognized ILC2 population.
Conclusions: The commonly used surface markers for human ILC2s leave a majority of type 2 cytokine-producing ILC2s unaccounted for. We identified top GATA3-correlated cell surface-expressed genes in human ILCs by RNA sequencing. These new surface markers, such as CD30 and TNFR2, identified a previously unrecognized human ILC2 population. This ILC2 population is likely to contribute to asthma.
Keywords: Asthma; cytokines; novel ILC2 population; type 2 innate lymphoid cells.
Copyright © 2020 American Academy of Allergy, Asthma & Immunology. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no competing financial interests.
Figures





Comment in
-
ILC2s: Are they what we think they are?J Allergy Clin Immunol. 2020 Aug;146(2):280-282. doi: 10.1016/j.jaci.2020.05.042. Epub 2020 Jun 12. J Allergy Clin Immunol. 2020. PMID: 32535133 Free PMC article. No abstract available.
References
-
- Klose CS, Artis D. Innate lymphoid cells as regulators of immunity, inflammation and tissue homeostasis. Nat Immunol 2016; 17:765–74. - PubMed
-
- Vivier E, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, et al. Innate Lymphoid Cells: 10 Years On. Cell 2018; 174:1054–66. - PubMed
-
- Mjosberg JM, Trifari S, Crellin NK, Peters CP, van Drunen CM, Piet B, et al. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat Immunol 2011; 12:1055–62. - PubMed
-
- Bjorklund AK, Forkel M, Picelli S, Konya V, Theorell J, Friberg D, et al. The heterogeneity of human CD127(+) innate lymphoid cells revealed by single-cell RNA sequencing. Nat Immunol 2016; 17:451–60. - PubMed
-
- Barlow JL, Peel S, Fox J, Panova V, Hardman CS, Camelo A, et al. IL-33 is more potent than IL-25 in provoking IL-13-producing nuocytes (type 2 innate lymphoid cells) and airway contraction. J Allergy Clin Immunol 2013; 132:933–41. - PubMed

