Synergistic activation of p53 by actinomycin D and nutlin-3a is associated with the upregulation of crucial regulators and effectors of innate immunity
- PMID: 32032660
- PMCID: PMC7126238
- DOI: 10.1016/j.cellsig.2020.109552
Synergistic activation of p53 by actinomycin D and nutlin-3a is associated with the upregulation of crucial regulators and effectors of innate immunity
Abstract
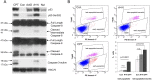
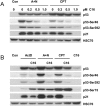
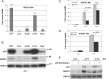
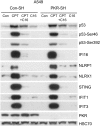
Actinomycin D and nutlin-3a (A + N) activate p53, partly through induction of phosphorylation on Ser392. The death of A549 cells induced by A + N morphologically resembles inflammation-inducing pyroptosis - cell destruction triggered by activated caspase-1. The treatment with A + N (or camptothecin) strongly upregulated caspase-1 and its two activators: IFI16 and NLRP1, however, caspase-1 activation was not detected. A549 cells may have been primed for pyroptosis, with the absence of a crucial trigger. The investigation of additional innate immunity elements revealed that A + N (or camptothecin) stimulated the expression of NLRX1, STING (stimulator of interferon genes) and two antiviral proteins, IFIT1 and IFIT3. IFI16 and caspase-1 are coded by p53-regulated genes which led us to investigate regulation of NLRP1, NLRX1, STING, IFIT1 and IFIT3 in p53-dependent mode. The upregulation of NLRP1, NLRX1 and STING was attenuated in p53 knockdown cells. The upsurge of the examined genes, and activation of p53, was inhibited by C16, an inhibitor of PKR kinase. PKR was tested due to its ability to phosphorylate p53 on Ser392. Surprisingly, C16 was active even in PKR knockdown cells. The ability of C16 to prevent activation of p53 and expression of innate immunity genes may be the source of its strong anti-inflammatory action. Moreover, cells exposed to A + N can influence neighboring cells in paracrine fashion, for instance, they shed ectodomain of COL17A1 protein and induce, in p53-dependent mode, the expression of gene for interleukin-7. Further, the activation of p53 also spurred the expression of SOCS1, an inhibitor of interferon triggered STAT1-dependent signaling. We conclude that, stimulation of p53 primes cells for the production of interferons (through upregulation of STING), and may activate negative-feedback within this signaling system by enhancing the production of SOCS1.
Keywords: IL7; Inflammation; Innate immunity; Interferon; Pyroptosis; p53.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare no conflict of interest.
Figures











References
-
- Zajkowicz A., Gdowicz-Kłosok A., Krześniak M., Ścieglińska D., Rusin M. Actinomycin D and nutlin-3a synergistically promote phosphorylation of p53 on serine 46 in cancer cell lines of different origin. Cell. Signal. 2015 Sep;27(9):1677–1687. - PubMed
-
- Zajkowicz A., Gdowicz-Kłosok A., Krześniak M., Janus P., Łasut B., Rusin M. The Alzheimer's disease-associated TREM2 gene is regulated by p53 tumor suppressor protein. Neurosci. Lett. 2018 Aug 10;681:62–67. - PubMed
-
- Choong M.L., Yang H., Lee M.A., Lane D.P. Specific activation of the p53 pathway by low dose actinomycin D: a new route to p53 based cyclotherapy. Cell Cycle. 2009 Sep 1;8(17):2810–2818. - PubMed
-
- Mantovani F., Tocco F., Girardini J., Smith P., Gasco M., Lu X., Crook T., Del Sal G. The prolyl isomerase Pin1 orchestrates p53 acetylation and dissociation from the apoptosis inhibitor iASPP. Nat. Struct. Mol. Biol. 2007 Oct;14(10):912–920. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

