The Susceptibility of Human Melanoma Cells to Infection with the Leningrad-16 Vaccine Strain of Measles Virus
- PMID: 32033013
- PMCID: PMC7077263
- DOI: 10.3390/v12020173
The Susceptibility of Human Melanoma Cells to Infection with the Leningrad-16 Vaccine Strain of Measles Virus
Abstract
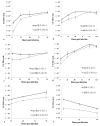
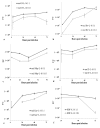
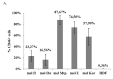
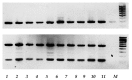
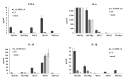
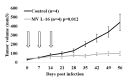
Oncolytic viruses, including live attenuated measles virus (MV) vaccine strains, have recently been shown as promising therapeutic agents against human malignancies. In this study, the oncolytic potential of the attenuated vaccine strain Leningrad-16 (L-16) of MV was evaluated in a panel of human metastatic melanoma cell lines. The L-16 measles virus was shown to replicate within melanoma cells mediating direct cell killing of tumor cells, although all melanoma cell lines varied in regard to their ability to respond to L-16 MV infection, as revealed by the different pattern of the Interferon Stimulated Gene expression, cytokine release and mechanisms of cell death. Furthermore, the statistically significant L-16 measles virus related tumor growth inhibition was demonstrated in a melanoma xenograft model. Therefore, L-16 MV represents an appealing oncolytic platform for target delivery of therapeutic genes along with other attenuated measles virus strains.
Keywords: cancer immunotherapy; measles virus; oncolytic viruses.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

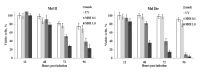
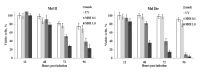





References
-
- Malishevskaya N.P., Sokolova A.V., Demidov L.V. The incidence of skin melanoma in the Russian Federation and federal districts. Med Counc. 2018;10:161–165. doi: 10.21518/2079-701X-2018-10-161-165. (In Russian) - DOI
-
- Matthews N.H., Li W.-Q., Qureshi A.A., Weinstock M.A., Cho E. Epidemiology of Melanoma. In: Ward W.H., Farma J.M., editors. Cutaneous Melanoma: Epidemiology and Therapy. Codon Publications; Brisbane, Australia: 2017. - PubMed
-
- Lacroix J., Kis Z., Josupeit R., Schlund F., Stroh-Dege A., Frank-Stöhr M., Leuchs B., Schlehofer J.R., Rommelaere J., Dinsart C. Preclinical Testing of an Oncolytic Parvovirus in Ewing Sarcoma: Protoparvovirus H-1 Induces Apoptosis and Lytic Infection In Vitro but Fails to Improve Survival In Vivo. Viruses. 2018;10:302. doi: 10.3390/v10060302. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

