Structural Features of Nucleoprotein CST/Shelterin Complex Involved in the Telomere Maintenance and Its Association with Disease Mutations
- PMID: 32033110
- PMCID: PMC7072152
- DOI: 10.3390/cells9020359
Structural Features of Nucleoprotein CST/Shelterin Complex Involved in the Telomere Maintenance and Its Association with Disease Mutations
Abstract
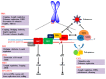
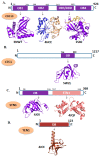
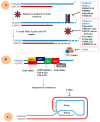
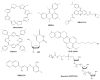
Telomere comprises the ends of eukaryotic linear chromosomes and is composed of G-rich (TTAGGG) tandem repeats which play an important role in maintaining genome stability, premature aging and onsets of many diseases. Majority of the telomere are replicated by conventional DNA replication, and only the last bit of the lagging strand is synthesized by telomerase (a reverse transcriptase). In addition to replication, telomere maintenance is principally carried out by two key complexes known as shelterin (TRF1, TRF2, TIN2, RAP1, POT1, and TPP1) and CST (CDC13/CTC1, STN1, and TEN1). Shelterin protects the telomere from DNA damage response (DDR) and regulates telomere length by telomerase; while, CST govern the extension of telomere by telomerase and C strand fill-in synthesis. We have investigated both structural and biochemical features of shelterin and CST complexes to get a clear understanding of their importance in the telomere maintenance. Further, we have analyzed ~115 clinically important mutations in both of the complexes. Association of such mutations with specific cellular fault unveils the importance of shelterin and CST complexes in the maintenance of genome stability. A possibility of targeting shelterin and CST by small molecule inhibitors is further investigated towards the therapeutic management of associated diseases. Overall, this review provides a possible direction to understand the mechanisms of telomere borne diseases, and their therapeutic intervention.
Keywords: CST complex; OB-fold proteins; Telomere replication; cancer; mutations; shelterin complex; small molecule inhibitors; structural genomics; telomere shortening.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
POT1-TPP1 telomere length regulation and disease.Comput Struct Biotechnol J. 2020 Jul 3;18:1939-1946. doi: 10.1016/j.csbj.2020.06.040. eCollection 2020. Comput Struct Biotechnol J. 2020. PMID: 32774788 Free PMC article. Review.
-
Structure and function of the telomeric CST complex.Comput Struct Biotechnol J. 2016 Apr 14;14:161-7. doi: 10.1016/j.csbj.2016.04.002. eCollection 2016. Comput Struct Biotechnol J. 2016. PMID: 27239262 Free PMC article. Review.
-
Structure of Tetrahymena telomerase-bound CST with polymerase α-primase.Nature. 2022 Aug;608(7924):813-818. doi: 10.1038/s41586-022-04931-7. Epub 2022 Jul 13. Nature. 2022. PMID: 35831498 Free PMC article.
-
Upregulation of shelterin and CST genes and longer telomeres are associated with unfavorable prognostic characteristics in prostate cancer.Cancer Genet. 2024 Jun;284-285:20-29. doi: 10.1016/j.cancergen.2024.03.006. Epub 2024 Mar 16. Cancer Genet. 2024. PMID: 38503134
-
POT1 recruits and regulates CST-Polα/primase at human telomeres.Cell. 2024 Jul 11;187(14):3638-3651.e18. doi: 10.1016/j.cell.2024.05.002. Epub 2024 Jun 4. Cell. 2024. PMID: 38838667 Free PMC article.
Cited by
-
POLIE suppresses telomerase-mediated telomere G-strand extension and helps ensure proper telomere C-strand synthesis in trypanosomes.Nucleic Acids Res. 2022 Feb 28;50(4):2036-2050. doi: 10.1093/nar/gkac023. Nucleic Acids Res. 2022. PMID: 35061898 Free PMC article.
-
The regulations of telomerase reverse transcriptase (TERT) in cancer.Cell Death Dis. 2024 Jan 26;15(1):90. doi: 10.1038/s41419-024-06454-7. Cell Death Dis. 2024. PMID: 38278800 Free PMC article. Review.
-
Non-canonical roles of canonical telomere binding proteins in cancers.Cell Mol Life Sci. 2021 May;78(9):4235-4257. doi: 10.1007/s00018-021-03783-0. Epub 2021 Feb 18. Cell Mol Life Sci. 2021. PMID: 33599797 Free PMC article. Review.
-
The stn1-sz2 Mutant Provides New Insight into the Impacts of Telomeric Cdc13-Stn1-Ten1 Dysfunction on Cell Cycle Progression.Cells. 2025 May 26;14(11):784. doi: 10.3390/cells14110784. Cells. 2025. PMID: 40497960 Free PMC article.
-
The Influence of Telomere-Related Gene Variants, Serum Levels, and Relative Leukocyte Telomere Length in Pituitary Adenoma Occurrence and Recurrence.Cancers (Basel). 2024 Feb 2;16(3):643. doi: 10.3390/cancers16030643. Cancers (Basel). 2024. PMID: 38339395 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

