Neuroimaging Biomarkers in SCA2 Gene Carriers
- PMID: 32033120
- PMCID: PMC7037189
- DOI: 10.3390/ijms21031020
Neuroimaging Biomarkers in SCA2 Gene Carriers
Abstract
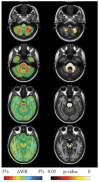
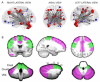
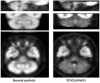
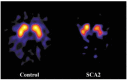
A variety of Magnetic Resonance (MR) and nuclear medicine (NM) techniques have been used in symptomatic and presymptomatic SCA2 gene carriers to explore,in vivo, the physiopathological biomarkers of the neurological dysfunctions characterizing the associated progressive disease that presents with a cerebellar syndrome, or less frequently, with a levodopa-responsive parkinsonian syndrome. Morphometry performed on T1-weighted images and diffusion MR imaging enable structural and microstructural evaluation of the brain in presymptomatic and symptomatic SCA2 gene carriers, in whom they show the typical pattern of olivopontocerebellar atrophy observed at neuropathological examination. Proton MR spectroscopy reveals, in the pons and cerebellum of SCA2 gene carriers,a more pronounced degree of abnormal neurochemical profile compared to other spinocerebellar ataxias with decreased NAA/Cr and Cho/Cr, increased mi/Cr ratios, and decreased NAA and increased mI concentrations. These neurochemical abnormalities are detectable also in presymtomatic gene carriers. Resting state functional MRI (rsfMRI) demonstrates decreased functional connectivity within the cerebellum and of the cerebellum with fronto-parietal cortices and basal ganglia in symptomatic SCA2 subjects. 18F-fluorodeoxyglucose Positron Emission Tomography (PET) shows a symmetric decrease of the glucose uptake in the cerebellar cortex, the dentate nucleus, the brainstem and the parahippocampal cortex. Single photon emission tomography and PET using several radiotracers have revealed almost symmetric nigrostriatal dopaminergic dysfunction irrespective of clinical signs of parkinsonism which are already present in presymtomatic gene carriers. Longitudinal small size studies have proven that morphometry and diffusion MR imaging can track neurodegeneration in SCA2, and hence serve as progression biomarkers. So far, such a capability has not been reported for proton MR spectroscopy, rsfMRI and NM techniques. A search for the best surrogate marker for future clinical trials represents the current challenge for the neuroimaging community.
Keywords: Key-words: spinocerebellar ataxia type 2; brainstem; cerebellum; magnetic resonance; nuclear medicine.
Conflict of interest statement
The authors declare no conflict of interest
Figures




References
-
- Stoyas C.A., La Spada A.R. The CAG-polyglutamine repeat diseases: A clinical, molecular, genetic, and pathophysiologic nosology. Handb. Clin. Neurol. 2018;147:143–170. - PubMed
-
- Rub U., Schols L., Paulson H., Auburger G., Kermer P., Jen J.C., Seidel K., Korf H.W., Deller T. Clinical features, neurogenetics and neuropathology of the polyglutamine spinocerebellar ataxias type 1, 2, 3, 6 and 7. Progr. Neurobiol. 2013;104:38–66. doi: 10.1016/j.pneurobio.2013.01.001. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

