Antiviral Ranpirnase TMR-001 Inhibits Rabies Virus Release and Cell-to-Cell Infection In Vitro
- PMID: 32033253
- PMCID: PMC7077210
- DOI: 10.3390/v12020177
Antiviral Ranpirnase TMR-001 Inhibits Rabies Virus Release and Cell-to-Cell Infection In Vitro
Abstract
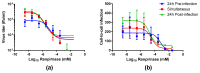
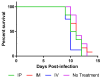
Currently, no rabies virus-specific antiviral drugs are available. Ranpirnase has strong antitumor and antiviral properties associated with its ribonuclease activity. TMR-001, a proprietary bulk drug substance solution of ranpirnase, was evaluated against rabies virus in three cell types: mouse neuroblastoma, BSR (baby hamster kidney cells), and bat primary fibroblast cells. When TMR-001 was added to cell monolayers 24 h preinfection, rabies virus release was inhibited for all cell types at three time points postinfection. TMR-001 treatment simultaneous with infection and 24 h postinfection effectively inhibited rabies virus release in the supernatant and cell-to-cell spread with 50% inhibitory concentrations of 0.2-2 nM and 20-600 nM, respectively. TMR-001 was administered at 0.1 mg/kg via intraperitoneal, intramuscular, or intravenous routes to Syrian hamsters beginning 24 h before a lethal rabies virus challenge and continuing once per day for up to 10 days. TMR-001 at this dose, formulation, and route of delivery did not prevent rabies virus transit from the periphery to the central nervous system in this model (n = 32). Further aspects of local controlled delivery of other active formulations or dose concentrations of TMR-001 or ribonuclease analogues should be investigated for this class of drugs as a rabies antiviral therapeutic.
Keywords: antiviral; hamster; lyssavirus; onconase; rabies virus; ranpirnase TMR-001.
Conflict of interest statement
Thomas Hodge, Luis Squiquera, and Jamie Sulley are or were employees of a company that develops antiviral therapeutics. The use of trade names and commercial sources are for identification only and do not imply endorsement by the U.S. Department of Health and Human Services. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention or the authors’ institutions
Figures


References
-
- Rupprecht C.E., Briggs D., Brown C.M., Franka R., Katz S.L., Kerr H.D., Lett S.M., Levis R., Meltzer M.I., Schaffner W., et al. Use of a reduced (4-dose) vaccine schedule for postexposure prophylaxis to prevent human rabies: Recommendations of the advisory committee on immunization practices. MMWR Recomm. Rep. 2010;59:1–9. - PubMed
-
- Hampson K., Coudeville L., Lembo T., Sambo M., Kieffer A., Attlan M., Barrat J., Blanton J.D., Briggs D.J., Cleaveland S., et al. Global Alliance for Rabies Control Partners for Rabies Prevention. Estimating the global burden of endemic canine rabies. PLoS Negl. Trop. Dis. 2015;9:e0003709. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

