VEGF/VEGF-R/RUNX2 Upregulation in Human Periodontal Ligament Stem Cells Seeded on Dual Acid Etched Titanium Disk
- PMID: 32033260
- PMCID: PMC7040902
- DOI: 10.3390/ma13030706
VEGF/VEGF-R/RUNX2 Upregulation in Human Periodontal Ligament Stem Cells Seeded on Dual Acid Etched Titanium Disk
Abstract
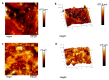
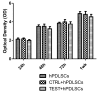
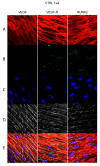
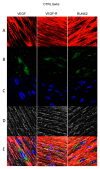
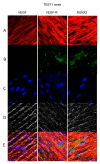
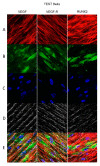
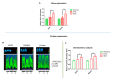
In restorative dentistry, the main implants characteristic is the ability to promote the osseointegration process as the result of interaction between angiogenesis and osteogenesis events. On the other hand, implants cytocompatibility remains a necessary feature for the success of surgery. The purpose of the current study was to investigate the interaction between human periodontal stem cells and two different types of titanium surfaces, to verify their cytocompatibility and cell adhesion ability, and to detect osteogenic and angiogenic markers, trough cell viability assay (MTT), Confocal Laser Scanning Microscopy (CLSM), scanning electron microscopy (SEM), and gene expression (RT-PCR). The titanium surfaces, machined (CTRL) and dual acid etched (TEST), tested in culture with human periodontal ligament stem cells (hPDLSCs), were previously treated in two different ways, in order to evaluate the effects of CTRL and TEST and define the best implant surface. Furthermore, the average surface roughness (Ra) of both titanium surfaces, CTRL and TEST, has been assessed through atomic force microscopy (AFM). The vascular endothelial growth factor (VEGF) and Runt-related transcription factor 2 (RUNX2) expressions have been analyzed by RT-PCR, WB analysis, and confocal laser scanning microscopy. Data evidenced that the different morphology and topography of the TEST disk increased cell growth, cell adhesion, improved osteogenic and angiogenic events, as well osseointegration process. For this reason, the TEST surface was more biocompatible than the CTRL disk surface.
Keywords: angiogenesis; cytocompatibility; osseointegration; osteogenesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Rajan T.S., Scionti D., Diomede F., Grassi G., Pollastro F., Piattelli A., Cocco L., Bramanti P., Mazzon E., Trubiani O. Gingival Stromal Cells as an In Vitro Model: Cannabidiol Modulates Genes Linked With Amyotrophic Lateral Sclerosis. J. Cell. Biochem. 2017;118:819–828. doi: 10.1002/jcb.25757. - DOI - PubMed
-
- Di Nisio C., De Colli M., di Giacomo V., Rapino M., Di Valerio V., Marconi G.D., Gallorini M., Di Giulio M., Cataldi A., Zara S. A dual role for beta1 integrin in an in vitro Streptococcus mitis/human gingival fibroblasts co-culture model in response to TEGDMA. Int. Endod. J. 2015;48:839–849. doi: 10.1111/iej.12379. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

