Nitric Oxide Synthase Is Involved in Follicular Development via the PI3K/AKT/FoxO3a Pathway in Neonatal and Immature Rats
- PMID: 32033275
- PMCID: PMC7070647
- DOI: 10.3390/ani10020248
Nitric Oxide Synthase Is Involved in Follicular Development via the PI3K/AKT/FoxO3a Pathway in Neonatal and Immature Rats
Abstract
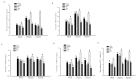
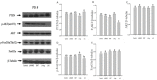
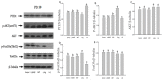
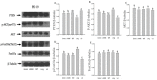
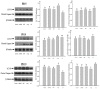
It is assumed that nitric oxide synthase and nitric oxide are involved in the regulation of female reproduction. This study aimed to assess the roles of nitric oxide synthase (NOS) in follicular development. The endothelial NOS (eNOS) inhibitor L-NAME, inducible NOS (iNOS) inhibitor S-Methylisothiourea (SMT) and NOS substrate L-arginine (L-Arg) were used in the NOS inhibition models in vivo. Neonatal female rats were treated with phosphate buffer saline (PBS, control), L-NAME (L-NG-Nitroarginine Methyl Ester, 40 mg/kg), SMT (S-Methylisothiourea, 10 mg/kg), L-NAME + SMT, or L-Arg (L-arginine, 50 mg/kg) via subcutaneous (SC) injection on a daily basis for 19 consecutive days, with the samples being collected on specific postnatal days (PD5, PD10, and PD19). The results indicated that the number of antral follicles, the activity of total-NOS, iNOS, neuronal NOS (nNOS), and eNOS, and the content of NO in the ovary were significantly (p < 0.05) increased in the L-Arg group at PD19, while those in L + S group were significantly (p < 0.05) decreased. Meanwhile, the ovarian expression in the L-Arg group in terms of p-AKT, p-FoxO3a, and LC3-II on PD19 were significantly (p < 0.05) upregulated, while the expressions of PTEN and cleaved Caspase-3 were (p < 0.05) downregulated as a result of NOS/NO generation, respectively. Therefore, the results suggest that NOS is possibly involved in the maturation of follicular development to puberty via the PI3K/ AKT/FoxO3a pathway, through follicular autophagia and apoptosis mechanisms.
Keywords: AKT; FoxO3a; PTEN; follicular development; nitric oxide synthase.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
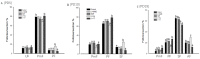
Similar articles
-
Regulation of tracheal ciliary beat frequency by nitric oxide synthase substrate L-arginine.ORL J Otorhinolaryngol Relat Spec. 2010;72(1):6-11. doi: 10.1159/000265683. Epub 2010 Jan 22. ORL J Otorhinolaryngol Relat Spec. 2010. PMID: 20110742
-
Activity of ovarian nitric oxide synthase (NOs) during ovulatory process in the rat: relationship with prostaglandins (PGs) production.Nitric Oxide. 1999 Aug;3(4):340-7. doi: 10.1006/niox.1999.0231. Nitric Oxide. 1999. PMID: 10444373
-
Nitric oxide in the bovine oviduct: influence on contractile activity and nitric oxide synthase isoforms localization.Theriogenology. 2012 Apr 15;77(7):1312-27. doi: 10.1016/j.theriogenology.2011.10.036. Epub 2012 Jan 5. Theriogenology. 2012. PMID: 22225690
-
Hormonal regulation of nitric oxide synthases and their cell-specific expression during follicular development in the rat ovary.Endocrinology. 1997 Jan;138(1):460-8. doi: 10.1210/endo.138.1.4884. Endocrinology. 1997. PMID: 8977436
-
Expression of nitric oxide synthase isoforms in different stages of buffalo (Bubalus bubalis) ovarian follicles: effect of nitric oxide on in vitro development of preantral follicle.Theriogenology. 2012 Jan 15;77(2):280-91. doi: 10.1016/j.theriogenology.2011.08.002. Epub 2011 Sep 14. Theriogenology. 2012. PMID: 21924465
Cited by
-
Expression Regulation and Physiological Role of Transcription Factor FOXO3a During Ovarian Follicular Development.Front Physiol. 2020 Nov 5;11:595086. doi: 10.3389/fphys.2020.595086. eCollection 2020. Front Physiol. 2020. PMID: 33250784 Free PMC article. Review.
-
Evaluation of Oxidative and Nitrosative Stress Markers Related To Inflammation in The Cumulus Cells and Follicular Fluid of Women Undergoing Intracytoplasmic Sperm Injection: A Prospective Study.Int J Fertil Steril. 2024 Feb 2;18(2):108-114. doi: 10.22074/ijfs.2023.559526.1342. Int J Fertil Steril. 2024. PMID: 38368512 Free PMC article.
-
Roles of Nitric Oxide in the Regulation of Reproduction: A Review.Front Endocrinol (Lausanne). 2021 Nov 19;12:752410. doi: 10.3389/fendo.2021.752410. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 34867795 Free PMC article. Review.
-
Nitric Oxide: Physiological Functions, Delivery, and Biomedical Applications.Adv Sci (Weinh). 2023 Oct;10(30):e2303259. doi: 10.1002/advs.202303259. Epub 2023 Aug 26. Adv Sci (Weinh). 2023. PMID: 37632708 Free PMC article. Review.
-
Uncovering Novel Protein Partners of Inducible Nitric Oxide Synthase in Human Testis.Biomolecules. 2024 Mar 24;14(4):388. doi: 10.3390/biom14040388. Biomolecules. 2024. PMID: 38672406 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

