Novel Superadsorbent Highly Porous Hydrogel Based on Arabic Gum and Acrylamide Grafts for Fast and Efficient Methylene Blue Removal
- PMID: 32033357
- PMCID: PMC7077498
- DOI: 10.3390/polym12020338
Novel Superadsorbent Highly Porous Hydrogel Based on Arabic Gum and Acrylamide Grafts for Fast and Efficient Methylene Blue Removal
Erratum in
-
Correction: Elbedwehy, A.M.; Atta, A.M. Novel Superadsorbent Highly Porous Hydrogel Based on Arabic Gum and Acrylamide Grafts for Fast and Efficient Methylene Blue Removal. Polymers 2020, 12, 338.Polymers (Basel). 2025 Jul 3;17(13):1861. doi: 10.3390/polym17131861. Polymers (Basel). 2025. PMID: 40647899 Free PMC article.
Abstract
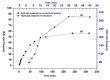
Environmental pollution with dyes released from industrial effluent is one of the major and most critical problems in the world. To alleviate this issue, advanced and safe materials with fast and highly efficient dye removal should be designed. Great attention has been paid recently to hydrogels based on polysaccharides such as Arabic Gum (AG) grafted with polyacrylamide (PAM) and polyacrylic acid (PAA). These materials combine the merits of natural polymers such as biodegradability and non-toxicity with the high adsorption ability of PAM and PAA towards cationic dyes such as methylene blue (MB). Many previous works have been done to enhance three-dimensional (3D) structure and swelling ability of the graft copolymers by using a crosslinking agent or even adding nanomaterials as a filler inside the hydrogel matrix. However, these additives may negatively affect the adsorption ability, and few previous studies could reach 2000 mg/g of maximum MB capacity removal within a good period of time. In our work, we synthesized partially hydrolyzed polyacrylamide grafted Arabic gum (AG-g-PAM/PAA) to have both amide and carboxylate groups. The modified water dissolved graft product undergoes water in oil (W/O) emulsion using paraffin oil as the continuous phase and Triton X-100 as a stabilizing agent; then, the system was inversed to oil in water (O/W) emulsion by increasing the shear mixing rate and cross-linked using Epichlorohydrin (ECH). The precipitated graft product showed hierarchically interconnected micro and macropores' sponge like shape with fast water swelling and high MB adsorption capacity (2300 mg g-1) after 45 min at near neutral pH conditions.
Keywords: arabic gum; emulsion inversion; hydrogel; porous; superadsorbent; water retention.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Yaseen D., Scholz M. Textile dye wastewater characteristics and constituents of synthetic effluents: A critical review. Int. J. Environ. Sci. Technol. 2019;16:1193–1226.
-
- Yu C., Wang F., Zhang C., Fu S., Lucia L.A. The synthesis and absorption dynamics of a lignin-based hydrogel for remediation of cationic dye-contaminated effluent. React. Funct. Polym. 2016;106:137–142.
-
- Benaïssa H. Effect of temperature on methylene blue sorption from aqueous solutions by almond peel: Experimental studies and modeling; Proceedings of the Thirteenth International Water Technology Conference (IWTC13); Hurghada, Egypt. 12–15 March 2009; pp. 377–393.
-
- Chequer F.D., de Oliveira G.A.R., Ferraz E.A., Cardoso J.C., Zanoni M.B., de Oliveira D.P. Textile dyes: Dyeing process and environmental impact. Eco-Friendly Text. Dye Finish. 2013;6:151–176.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

