Glucose as a Major Antioxidant: When, What for and Why It Fails?
- PMID: 32033390
- PMCID: PMC7070274
- DOI: 10.3390/antiox9020140
Glucose as a Major Antioxidant: When, What for and Why It Fails?
Abstract
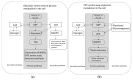
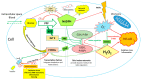
A human organism depends on stable glucose blood levels in order to maintain its metabolic needs. Glucose is considered to be the most important energy source, and glycolysis is postulated as a backbone pathway. However, when the glucose supply is limited, ketone bodies and amino acids can be used to produce enough ATP. In contrast, for the functioning of the pentose phosphate pathway (PPP) glucose is essential and cannot be substituted by other metabolites. The PPP generates and maintains the levels of nicotinamide adenine dinucleotide phosphate (NADPH) needed for the reduction in oxidized glutathione and protein thiols, the synthesis of lipids and DNA as well as for xenobiotic detoxification, regulatory redox signaling and counteracting infections. The flux of glucose into a PPP-particularly under extreme oxidative and toxic challenges-is critical for survival, whereas the glycolytic pathway is primarily activated when glucose is abundant, and there is lack of NADP+ that is required for the activation of glucose-6 phosphate dehydrogenase. An important role of glycogen stores in resistance to oxidative challenges is discussed. Current evidences explain the disruptive metabolic effects and detrimental health consequences of chronic nutritional carbohydrate overload, and provide new insights into the positive metabolic effects of intermittent fasting, caloric restriction, exercise, and ketogenic diet through modulation of redox homeostasis.
Keywords: NADPH; glucose; glycogen; glycolysis; insulin resistance; pentose phosphate pathway; redox balance; stress resistance.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Aronoff S.L., Berkowitz K., Shreiner B., Want L. Glucose metabolism and regulation: Beyond insulin and glucagon. Diabetes Spectr. 2004;17:183–190. doi: 10.2337/diaspect.17.3.183. - DOI
Publication types
LinkOut - more resources
Full Text Sources

