Exposure of CuO Nanoparticles Contributes to Cellular Apoptosis, Redox Stress, and Alzheimer's Aβ Amyloidosis
- PMID: 32033400
- PMCID: PMC7038189
- DOI: 10.3390/ijerph17031005
Exposure of CuO Nanoparticles Contributes to Cellular Apoptosis, Redox Stress, and Alzheimer's Aβ Amyloidosis
Abstract
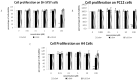
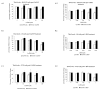
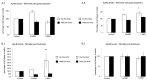
Fe2O3, CuO and ZnO nanoparticles (NP) have found various industrial and biomedical applications. However, there are growing concerns among the general public and regulators about their potential environmental and health impacts as their physio-chemical interaction with biological systems and toxic responses of the latter are complex and not well understood. Herein we first reported that human SH-SY5Y and H4 cells and rat PC12 cell lines displayed concentration-dependent neurotoxic responses to insults of CuO nanoparticles (CuONP), but not to Fe2O3 nanoparticles (Fe2O3NP) or ZnO nanoparticles (ZnONP). This study provides evidence that CuONP induces neuronal cell apoptosis, discerns a likely p53-dependent apoptosis pathway and builds out the relationship between nanoparticles and Alzheimer's disease (AD) through the involvement of reactive oxygen species (ROS) and increased Aβ levels in SH-SY5Y and H4 cells. Our results implicate that exposure to CuONP may be an environmental risk factor for AD. For public health concerns, regulation for environmental or occupational exposure of CuONP are thus warranted given AD has already become a pandemic.
Keywords: Alzheimer’s disease; Aβ; apoptosis; engineered nanomaterials; nanoparticles; reactive oxygen species.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







Similar articles
-
Uptake of silica nanoparticles: neurotoxicity and Alzheimer-like pathology in human SK-N-SH and mouse neuro2a neuroblastoma cells.Toxicol Lett. 2014 Aug 17;229(1):240-9. doi: 10.1016/j.toxlet.2014.05.009. Epub 2014 May 14. Toxicol Lett. 2014. PMID: 24831964
-
Exposure to CuO Nanoparticles Mediates NFκB Activation and Enhances Amyloid Precursor Protein Expression.Biomedicines. 2020 Feb 27;8(3):45. doi: 10.3390/biomedicines8030045. Biomedicines. 2020. PMID: 32120908 Free PMC article.
-
Amentoflavone suppresses amyloid β1-42 neurotoxicity in Alzheimer's disease through the inhibition of pyroptosis.Life Sci. 2019 Dec 15;239:117043. doi: 10.1016/j.lfs.2019.117043. Epub 2019 Nov 10. Life Sci. 2019. PMID: 31722188
-
Can CuO nanoparticles lead to epigenetic regulation of antioxidant enzyme system?J Appl Toxicol. 2017 Jan;37(1):84-91. doi: 10.1002/jat.3392. Epub 2016 Sep 30. J Appl Toxicol. 2017. PMID: 27687502 Review.
-
The redox chemistry of the Alzheimer's disease amyloid beta peptide.Biochim Biophys Acta. 2007 Aug;1768(8):1976-90. doi: 10.1016/j.bbamem.2007.02.002. Epub 2007 Feb 9. Biochim Biophys Acta. 2007. PMID: 17433250 Review.
Cited by
-
Self-Therapeutic Nanomaterials: Applications in Biology and Medicine.Mater Today (Kidlington). 2023 Jan-Feb;62:190-224. doi: 10.1016/j.mattod.2022.11.007. Epub 2022 Nov 29. Mater Today (Kidlington). 2023. PMID: 36938366 Free PMC article.
-
Engineered Nanoparticle-Protein Interactions Influence Protein Structural Integrity and Biological Significance.Nanomaterials (Basel). 2022 Apr 5;12(7):1214. doi: 10.3390/nano12071214. Nanomaterials (Basel). 2022. PMID: 35407332 Free PMC article. Review.
-
Synthesis of chitosan/PVA/copper oxide nanocomposite using Anacardium occidentale extract and evaluating its antioxidant, antibacterial, anti-inflammatory and cytotoxic activities.Sci Rep. 2025 Jan 31;15(1):3931. doi: 10.1038/s41598-025-87932-6. Sci Rep. 2025. PMID: 39890965 Free PMC article.
-
Antimicrobial Nano-Agents: The Copper Age.ACS Nano. 2021 Apr 27;15(4):6008-6029. doi: 10.1021/acsnano.0c10756. Epub 2021 Apr 1. ACS Nano. 2021. PMID: 33792292 Free PMC article. Review.
-
Alzheimer's Disease Pathology and Assistive Nanotheranostic Approaches for Its Therapeutic Interventions.Int J Mol Sci. 2024 Sep 7;25(17):9690. doi: 10.3390/ijms25179690. Int J Mol Sci. 2024. PMID: 39273645 Free PMC article. Review.
References
-
- ASTM E2456-06, Standard Terminology Relating to Nanotechnology. [(accessed on 23 January 2020)]; Available online: https://statnano.com/standard/astm/1121.
-
- Quintana A., Raczka E., Piehler L., Lee I., Myc A., Majoros I., Patri A.K., Thomas T., Mule J., Baker J.R., Jr. Design and function of a dendrimer-based therapeutic nanodevice targeted to tumor cells through thefolate receptor. Pharm. Res. 2002;19:1310–1316. doi: 10.1023/A:1020398624602. - DOI - PubMed
-
- Morawski A.M., Winter P.M., Crowder K.C., Caruthers S.D., Fuhrhop R.W., Scott M.J., Robertson J.D., Abendschein D.R., Lanza G.M., Wickline S.A. Targeted nanoparticles for quantitative imaging of sparse molecular epitopes with MRI. Magn. Reson. Med. 2004;51:480–486. doi: 10.1002/mrm.20010. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

