Regulation of Osteoclast Differentiation at Multiple Stages by Protein Kinase D Family Kinases
- PMID: 32033440
- PMCID: PMC7036879
- DOI: 10.3390/ijms21031056
Regulation of Osteoclast Differentiation at Multiple Stages by Protein Kinase D Family Kinases
Abstract
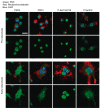
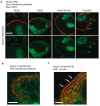
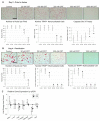
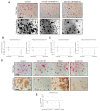
Balanced osteoclast and osteoblast activity is necessary for skeletal health, whereas unbalanced osteoclast activity causes bone loss in many skeletal conditions. A better understanding of pathways that regulate osteoclast differentiation and activity is necessary for the development of new therapies to better manage bone resorption. The roles of Protein Kinase D (PKD) family of serine/threonine kinases in osteoclasts have not been well characterized. In this study we use immunofluorescence analysis to reveal that PKD2 and PKD3, the isoforms expressed in osteoclasts, are found in the nucleus and cytoplasm, the mitotic spindle and midbody, and in association with the actin belt. We show that PKD inhibitors CRT0066101 and CID755673 inhibit several distinct aspects of osteoclast formation. Treating bone marrow macrophages with lower doses of the PKD inhibitors had little effect on M-CSF + RANKL-dependent induction into committed osteoclast precursors, but inhibited their motility and subsequent differentiation into multinucleated mature osteoclasts, whereas higher doses of the PKD inhibitors induced apoptosis of the preosteoclasts. Treating post-fusion multinucleated osteoclasts with the inhibitors disrupted the osteoclast actin belts and impaired their resorptive activity. In conclusion, these data implicate PKD kinases as positive regulators of osteoclasts, which are essential for multiple distinct processes throughout their formation and function.
Keywords: actin cytoskeleton; bone resorption; cellular differentiation; osteoclasts; protein kinase D.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

