Heterogeneous Responses of Gastric Cancer Cell Lines to Tenovin-6 and Synergistic Effect with Chloroquine
- PMID: 32033497
- PMCID: PMC7072542
- DOI: 10.3390/cancers12020365
Heterogeneous Responses of Gastric Cancer Cell Lines to Tenovin-6 and Synergistic Effect with Chloroquine
Abstract
Gastric cancer (GC) is the fifth most frequently diagnosed cancer and the third leading cause of cancer death. Approximately 15% of GC is associated with Epstein-Barr virus (EBV). GC is largely incurable with a dismal five-year survival rate. There is an urgent need to identify new therapeutic agents for the treatment of GC. Tenovin-6 was initially identified as a p53 activator, but it was later found to inhibit autophagy flux, and the protein deacetylase activity of sirtuins. Tenovin-6 shows promising therapeutic effect in various malignancies. However, it remains unknown whether Tenovin-6 is effective for GC. In this study, we found that EBV-positive and -negative GC cell lines were sensitive to Tenovin-6 but with different response times and doses. Tenovin-6 suppressed anchorage-independent growth of GC cells. Tenovin-6 induced different levels of apoptosis and phases of cell-cycle arrest depending on the cell lines with some manifesting gap 1 (G1) and others showing synthesis (S) phase cell-cycle arrest. Mechanistically, Tenovin-6 induced autophagy or p53 activation in GC cells depending on the status of TP53 gene. However, initiation of autophagy following treatment with Tenovin-6 conferred some protective effect on numerous cells. Combined treatment with Tenovin-6 and autophagy inhibitor chloroquine increased the cytotoxic effect by inducing microtubule-associated protein 1 light chain 3B (LC3B)-II accumulation, and by enhancing apoptosis and cell-cycle arrest. These results indicated that Tenovin-6 can be used as a potential therapeutic agent for GC, but the genetic background of the cancer cells might determine the response and mechanism of action. Treatment with Tenovin-6 alone or in combination with chloroquine could be a promising therapeutic approach for GC.
Keywords: Epstein–Barr virus (EBV); Tenovin-6; autophagy; chloroquine; gastric cancer; p53 activation.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
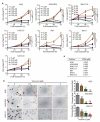
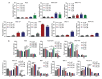




Similar articles
-
Tenovin-6 inhibits proliferation and survival of diffuse large B-cell lymphoma cells by blocking autophagy.Oncotarget. 2017 Feb 28;8(9):14912-14924. doi: 10.18632/oncotarget.14741. Oncotarget. 2017. PMID: 28118604 Free PMC article.
-
The sirtuin inhibitor tenovin-6 upregulates death receptor 5 and enhances cytotoxic effects of 5-fluorouracil and oxaliplatin in colon cancer cells.Oncol Res. 2013;21(3):155-64. doi: 10.3727/096504013X13854886566598. Oncol Res. 2013. PMID: 24512730
-
Tenovin-6 induces the SIRT-independent cell growth suppression and blocks autophagy flux in canine hemangiosarcoma cell lines.Exp Cell Res. 2020 Mar 1;388(1):111810. doi: 10.1016/j.yexcr.2019.111810. Epub 2019 Dec 28. Exp Cell Res. 2020. PMID: 31891684
-
Tenovin-6 impairs autophagy by inhibiting autophagic flux.Cell Death Dis. 2017 Feb 9;8(2):e2608. doi: 10.1038/cddis.2017.25. Cell Death Dis. 2017. PMID: 28182004 Free PMC article.
-
Pathogenesis of Gastric Cancer.Helicobacter. 2015 Sep;20 Suppl 1:30-5. doi: 10.1111/hel.12254. Helicobacter. 2015. PMID: 26372822 Review.
Cited by
-
Chloroquine against malaria, cancers and viral diseases.Drug Discov Today. 2020 Sep 16;25(11):2012-22. doi: 10.1016/j.drudis.2020.09.010. Online ahead of print. Drug Discov Today. 2020. PMID: 32947043 Free PMC article. Review.
-
Drug Resistance and Novel Therapies in Cancers in 2020.Cancers (Basel). 2023 Jan 24;15(3):717. doi: 10.3390/cancers15030717. Cancers (Basel). 2023. PMID: 36765674 Free PMC article.
-
MKG-GC: A multi-task learning-based knowledge graph construction framework with personalized application to gastric cancer.Comput Struct Biotechnol J. 2024 Mar 27;23:1339-1347. doi: 10.1016/j.csbj.2024.03.021. eCollection 2024 Dec. Comput Struct Biotechnol J. 2024. PMID: 38585647 Free PMC article.
-
Triangular Relationship between p53, Autophagy, and Chemotherapy Resistance.Int J Mol Sci. 2020 Nov 26;21(23):8991. doi: 10.3390/ijms21238991. Int J Mol Sci. 2020. PMID: 33256191 Free PMC article. Review.
-
Virtual Screening in the Identification of Sirtuins' Activity Modulators.Molecules. 2022 Sep 1;27(17):5641. doi: 10.3390/molecules27175641. Molecules. 2022. PMID: 36080416 Free PMC article. Review.
References
-
- McCarthy A.R., Pirrie L., Hollick J.J., Ronseaux S., Campbell J., Higgins M., Staples O.D., Tran F., Slawin A.M., Lain S., et al. Synthesis and biological characterisation of sirtuin inhibitors based on the tenovins. Bioorg Med. Chem. 2012;20:1779–1793. doi: 10.1016/j.bmc.2012.01.001. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

