The methanol extract of Guettarda speciosa Linn. Ameliorates acute lung injury in mice
- PMID: 32033557
- PMCID: PMC7076890
- DOI: 10.1186/s12906-020-2828-6
The methanol extract of Guettarda speciosa Linn. Ameliorates acute lung injury in mice
Abstract
Background: Guettarda speciosa is mainly found in tropical areas in Asia. Although G. speciosa is traditionally used to treat some of the inflammatory disorders, the experimental evidence supporting the anti-inflammatory effect of G. speciosa is limited. Here, we sought to obtain evidence that G. speciosa has anti-inflammatory activity using an acute lung injury (ALI) mouse model and to explore possible underlying mechanisms for the activity.
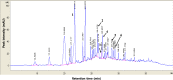
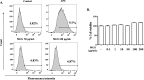
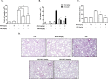
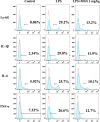
Methods: The methanol extract of G. speciosa Linn. (MGS) was fingerprinted by HPLC. Cytotoxicity was determined by MTT and flow cytometer. As for an ALI mouse model, C57BL/6 mice received an intratracheal (i.t.) injection of lipopolysaccharide (LPS). The effects of MGS on lung inflammation in the ALI mice were assessed by differential cell counting and FACS of inflammatory cells and hematoxylin and eosin staining of lung tissue. Proteins were analyzed by immunoprecipitation and immunoblotting, and gene expression was by real-time qPCR. Neutrophil elastase activity was measured by ELISA.
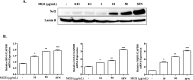
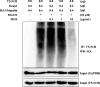
Results: MGS did not cause metabolic disarray or produce reactive oxygen species that could induce cytotoxicity. Similar to ALI patients, C57BL/6 mice that received an i.t. LPS developed a high level of neutrophils, increased pro-inflammatory cytokines, and inflicted tissue damage in the lung, which was suppressed by i.t. MGS administered at 2 h after LPS. Mechanistically, MGS activated Nrf2, which was related to MGS interrupting the ubiquitin-dependent degradation of Nrf2. MGS suppressed the nuclear localization of NF-κB induced by LPS, suggesting the inhibition of NF-κB activity. Furthermore, MGS inhibited the enzymatic activity of neutrophil elastase.
Conclusion: MGS could suppress lung inflammation in an ALI mouse model, the effect of which could be attributed to multiple mechanisms, including the activation of Nrf2 and the suppression of NF-κB and neutrophil elastase enzymatic activity by MGS.
Keywords: Acute lung injury; Anti-inflammation; Guettarda speciosa Linn.; NF-κB; Neutrophil elastase; Nrf2.
Conflict of interest statement
The authors do not have a commercial or other association that might have a conflict of interest.
Figures

Similar articles
-
Suppression of lung inflammation by the methanol extract of Spilanthes acmella Murray is related to differential regulation of NF-κB and Nrf2.J Ethnopharmacol. 2018 May 10;217:89-97. doi: 10.1016/j.jep.2018.02.011. Epub 2018 Feb 10. J Ethnopharmacol. 2018. PMID: 29432855
-
Therapeutic effect of Mahaenggamseok-tang on neutrophilic lung inflammation is associated with NF-κB suppression and Nrf2 activation.J Ethnopharmacol. 2016 Nov 4;192:486-495. doi: 10.1016/j.jep.2016.09.040. Epub 2016 Sep 20. J Ethnopharmacol. 2016. PMID: 27660010
-
Downregulated microRNA-27b attenuates lipopolysaccharide-induced acute lung injury via activation of NF-E2-related factor 2 and inhibition of nuclear factor κB signaling pathway.J Cell Physiol. 2019 May;234(5):6023-6032. doi: 10.1002/jcp.27187. Epub 2018 Dec 24. J Cell Physiol. 2019. PMID: 30584668
-
Potential therapeutic interventions of plant-derived isoflavones against acute lung injury.Int Immunopharmacol. 2021 Dec;101(Pt A):108204. doi: 10.1016/j.intimp.2021.108204. Epub 2021 Oct 4. Int Immunopharmacol. 2021. PMID: 34619497 Review.
-
Potential effects of medicinal plants and secondary metabolites on acute lung injury.Biomed Res Int. 2013;2013:576479. doi: 10.1155/2013/576479. Epub 2013 Oct 9. Biomed Res Int. 2013. PMID: 24224172 Free PMC article. Review.
Cited by
-
Guettarda crispiflora Vahl Methanol Extract Ameliorates Acute Lung Injury and Gastritis by Suppressing Src Phosphorylation.Plants (Basel). 2022 Dec 16;11(24):3560. doi: 10.3390/plants11243560. Plants (Basel). 2022. PMID: 36559672 Free PMC article.
-
Alismol Purified from the Tuber of Alisma orientale Relieves Acute Lung Injury in Mice via Nrf2 Activation.Int J Mol Sci. 2023 Oct 25;24(21):15573. doi: 10.3390/ijms242115573. Int J Mol Sci. 2023. PMID: 37958556 Free PMC article.
References
-
- Rodger WE, David JL, Trevor B. Encyclopedia of Australian Plants Suitable for Cultivation. Port Melbourne: Lothian Press; 1990. p. 162.
-
- Gandhimathi R, Saravana KA, Kumar KKS, Kusuma PK, Uma MJ. Pharmacological studies of anti-diarrhoeal activity of Guettarda speciosa (L.) in experimental animals. J Pharm Sci Res. 2009;2:61–67.
-
- Le HTT, Cho YC, Cho S. methanol extract of Guettarda speciosa Linn. Inhibits the production of inflammatory mediators through the inactivation of Syk and JNK in macrophages. Int J Mol Med. 2018;41(3):1783–1791. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

