Prognostic Testing and Treatment Patterns in Chronic Lymphocytic Leukemia in the Era of Novel Targeted Therapies: Results From the informCLL Registry
- PMID: 32033927
- PMCID: PMC7890939
- DOI: 10.1016/j.clml.2019.10.009
Prognostic Testing and Treatment Patterns in Chronic Lymphocytic Leukemia in the Era of Novel Targeted Therapies: Results From the informCLL Registry
Abstract
Introduction: The therapeutic landscape for chronic lymphocytic leukemia (CLL) has significantly shifted with the approval of novel agents. Understanding current prognostic testing and treatment practices in this new era is critical. Beginning enrollment in 2015, informCLL is the first United States-based real-world, prospective, observational registry that initiated enrollment after approval of novel agents.
Patients and methods: Eligible patients were age ≥ 18 years, started CLL treatment within 30 days of enrollment, and provided consent. For this planned interim analysis, treatments were classified into 5 groups: ibrutinib, chemoimmunotherapy, chemotherapy, immunotherapy, and other novel agents.
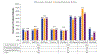
Results: Frequency of prognostic testing and treatment patterns are reported among 840 patients (459 previously untreated; 381 relapsed/refractory), enrolled largely (96%) from community practice settings. Testing for chromosomal abnormalities by fluorescence in situ hybridization, TP53 mutation, or IGHV mutation status occurred infrequently among all patients (31%, 11%, and 11%, respectively). Chemoimmunotherapy was the most common treatment in previously untreated patients (42%), whereas ibrutinib was the most common treatment among relapsed/refractory patients (51%). Of patients who tested positive for del(17p) or TP53 mutation, 34% and 26% received chemoimmunotherapy, respectively. Among patients who did not have fluorescence in situ hybridization or TP53 mutation testing prior to enrollment, 33% and 32% received chemoimmunotherapy, respectively.
Conclusion: Our findings indicate that prognostic testing rates were poor, and approximately one-third of high-risk patients (del[17p] and TP53) received chemoimmunotherapy, which is not aligned with current CLL treatment recommendations. This represents an opportunity to educate and alert health care professionals about the necessity of prognostic testing to guide optimal CLL treatment decisions.
Keywords: Chemoimmunotherapy; Ibrutinib; Novel agents; Prognostic marker; Real-world registry.
Copyright © 2019 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The remaining authors have stated that they have no conflicts of interest.
Figures
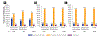

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

