Emergence of Fluoroquinolone-Resistant Campylobacter jejuni and Campylobacter coli among Australian Chickens in the Absence of Fluoroquinolone Use
- PMID: 32033955
- PMCID: PMC7117913
- DOI: 10.1128/AEM.02765-19
Emergence of Fluoroquinolone-Resistant Campylobacter jejuni and Campylobacter coli among Australian Chickens in the Absence of Fluoroquinolone Use
Abstract
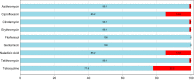
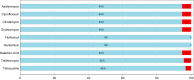
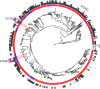
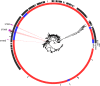
In a structured survey of all major chicken-meat producers in Australia, we investigated the antimicrobial resistance (AMR) and genomic characteristics of Campylobacter jejuni (n = 108) and C. coli (n = 96) from cecal samples of chickens at slaughter (n = 200). The majority of the C. jejuni (63%) and C. coli (86.5%) samples were susceptible to all antimicrobials. Fluoroquinolone resistance was detected among both C. jejuni (14.8%) and C. coli (5.2%), although this only included three sequence types (STs) and one ST, respectively. Multidrug resistance among strains of C. jejuni (0.9%) and C. coli (4.1%) was rare, and fluoroquinolone resistance, when present, was never accompanied by resistance to any other agent. Comparative genome analysis demonstrated that Australian isolates were found dispersed on different branches/clusters within the international collection. The major fluoroquinolone-resistant STs of C. jejuni (ST7323, ST2083, and ST2343) and C. coli (ST860) present in Australian chickens were similar to those of international isolates and have been reported previously in humans and animals overseas. The detection of a subpopulation of Campylobacter isolates exclusively resistant to fluoroquinolone was unexpected since most critically important antimicrobials such as fluoroquinolones are excluded from use in Australian livestock. A number of factors, including the low level of resistance to other antimicrobials, the absence of fluoroquinolone use, the adoption of measures for preventing spread of contagion between flocks, and particularly the genomic identities of isolates, all point to humans, pest species, or wild birds as being the most plausible source of organisms. This study also demonstrates the need for vigilance in the form of surveillance for AMR based on robust sampling to manage AMR risks in the food chain.IMPORTANCECampylobacter is one of the most common causes of gastroenteritis in humans, with infections frequently resulting from exposure to undercooked poultry products. Although human illness is typically self-limiting, a minority of cases do require antimicrobial therapy. Ensuring that Campylobacter originating from meat chickens does not acquire resistance to fluoroquinolones is therefore a valuable outcome for public health. Australia has never legalized the use of fluoroquinolones in commercial chickens and until now fluoroquinolone-resistant Campylobacter has not been detected in the Australian poultry. This structured survey of meat chickens derived from all major Australian producers describes the unexpected emergence of fluoroquinolone resistance in Campylobacter jejuni and C. coli Genetic characterization suggests that these isolates may have evolved outside the Australian poultry sector and were introduced into poultry by humans, pest species, or wild birds. The findings dramatically underline the critical role of biosecurity in the overall fight against antimicrobial resistance.
Keywords: AMR; Australia; Campylobacter; Campylobacter coli; Campylobacter jejuni; antimicrobial resistance; chicken; fluoroquinolone; genome analysis; livestock.
Copyright © 2020 Abraham et al.
Figures
References
-
- Müllner P, Collins-Emerson JM, Midwinter AC, Carter P, Spencer SE, van der Logt P, Hathaway S, French NP. 2010. Molecular epidemiology of Campylobacter jejuni in a geographically isolated country with a uniquely structured poultry industry. Appl Environ Microbiol 76:2145–2154. doi:10.1128/AEM.00862-09. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

