Recent advances in functional genomics for parasitic nematodes of mammals
- PMID: 32034038
- PMCID: PMC7790190
- DOI: 10.1242/jeb.206482
Recent advances in functional genomics for parasitic nematodes of mammals
Abstract
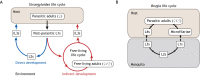
Human-parasitic nematodes infect over a quarter of the world's population and are a major cause of morbidity in low-resource settings. Currently available treatments have not been sufficient to eliminate infections in endemic areas, and drug resistance is an increasing concern, making new treatment options a priority. The development of new treatments requires an improved understanding of the basic biology of these nematodes. Specifically, a better understanding of parasitic nematode development, reproduction and behavior may yield novel drug targets or new opportunities for intervention such as repellents or traps. Until recently, our ability to study parasitic nematode biology was limited because few tools were available for their genetic manipulation. This is now changing as a result of recent advances in the large-scale sequencing of nematode genomes and the development of new techniques for their genetic manipulation. Notably, skin-penetrating gastrointestinal nematodes in the genus Strongyloides are now amenable to transgenesis, RNAi and CRISPR/Cas9-mediated targeted mutagenesis, positioning the Strongyloides species as model parasitic nematode systems. A number of other mammalian-parasitic nematodes, including the giant roundworm Ascaris suum and the tissue-dwelling filarial nematode Brugia malayi, are also now amenable to transgenesis and/or RNAi in some contexts. Using these tools, recent studies of Strongyloides species have already provided insight into the molecular pathways that control the developmental decision to form infective larvae and that drive the host-seeking behaviors of infective larvae. Ultimately, a mechanistic understanding of these processes could lead to the development of new avenues for nematode control.
Keywords: CRISPR; Parasitic helminth; Parasitic nematode; RNAi; Strongyloides; Transgenesis.
© 2020. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures

References
-
- Ahringer J. (2006). Reverse genetics. In WormBook (ed. The C. elegans Research Community) 1-43. 10.1895/wormbook.1.47.1, www.wormbook.org. - DOI
-
- Atif M., Smith J. J., Estrada-Mondragon A., Xiao X., Salim A. A., Capon R. J., Lynch J. W. and Keramidas A. (2019). GluClR-mediated inhibitory postsynaptic currents reveal targets for ivermectin and potential mechanisms of ivermectin resistance. PLoS Pathog. 15, e1007570 10.1371/journal.ppat.1007570 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

