Specialization of mucosal immunoglobulins in pathogen control and microbiota homeostasis occurred early in vertebrate evolution
- PMID: 32034088
- PMCID: PMC7296778
- DOI: 10.1126/sciimmunol.aay3254
Specialization of mucosal immunoglobulins in pathogen control and microbiota homeostasis occurred early in vertebrate evolution
Abstract
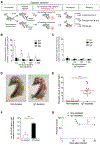
Although mammalian secretory immunoglobulin A (sIgA) targets mucosal pathogens for elimination, its interaction with the microbiota also enables commensal colonization and homeostasis. This paradoxical requirement in the control of pathogens versus microbiota raised the question of whether mucosal (secretory) Igs (sIgs) evolved primarily to protect mucosal surfaces from pathogens or to maintain microbiome homeostasis. To address this central question, we used a primitive vertebrate species (rainbow trout) in which we temporarily depleted its mucosal Ig (sIgT). Fish devoid of sIgT became highly susceptible to a mucosal parasite and failed to develop compensatory IgM responses against it. IgT depletion also induced a profound dysbiosis marked by the loss of sIgT-coated beneficial taxa, expansion of pathobionts, tissue damage, and inflammation. Restitution of sIgT levels in IgT-depleted fish led to a reversal of microbial translocation and tissue damage, as well as to restoration of microbiome homeostasis. Our findings indicate that specialization of sIgs in pathogen and microbiota control occurred concurrently early in evolution, thus revealing primordially conserved principles under which primitive and modern sIgs operate in the control of microbes at mucosal surfaces.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Figures


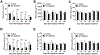




References
-
- Woof JM, Mestecky J, Mucosal immunoglobulins. Immunological reviews 206, 64–82 (2005). - PubMed
-
- Macpherson AJ, Yilmaz B, Limenitakis JP, Ganal-Vonarburg SC, IgA Function in Relation to the Intestinal Microbiota. Annual review of immunology 36, 359–381 (2018). - PubMed
-
- Dann SM, Manthey CF, Le C, Miyamoto Y, Gima L, Abrahim A, Cao AT, Hanson EM, Kolls JK, Raz E, Cong Y, Eckmann L, IL-17A promotes protective IgA responses and expression of other potential effectors against the lumen-dwelling enteric parasite Giardia. Experimental parasitology 156, 68–78 (2015). - PMC - PubMed
-
- Renegar KB, Small PA Jr., Boykins LG, Wright PF, Role of IgA versus IgG in the control of influenza viral infection in the murine respiratory tract Journal of immunology (Baltimore, Md. : 1950) 173, 1978–1986 (2004). - PubMed
-
- Sun K, Johansen FE, Eckmann L, Metzger DW, An important role for polymeric Ig receptor-mediated transport of IgA in protection against Streptococcus pneumoniae nasopharyngeal carriage Journal of immunology (Baltimore, Md. : 1950) 173, 4576–4581 (2004). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

