Anti-Factor B Antibodies and Acute Postinfectious GN in Children
- PMID: 32034108
- PMCID: PMC7191928
- DOI: 10.1681/ASN.2019080851
Anti-Factor B Antibodies and Acute Postinfectious GN in Children
Abstract
Background: The pathophysiology of the leading cause of pediatric acute nephritis, acute postinfectious GN, including mechanisms of the pathognomonic transient complement activation, remains uncertain. It shares clinicopathologic features with C3 glomerulopathy, a complement-mediated glomerulopathy that, unlike acute postinfectious GN, has a poor prognosis.
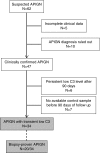
Methods: This retrospective study investigated mechanisms of complement activation in 34 children with acute postinfectious GN and low C3 level at onset. We screened a panel of anticomplement protein autoantibodies, carried out related functional characterization, and compared results with those of 60 children from the National French Registry who had C3 glomerulopathy and persistent hypocomplementemia.
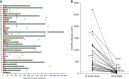
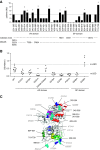
Results: All children with acute postinfectious GN had activation of the alternative pathway of the complement system. At onset, autoantibodies targeting factor B (a component of the alternative pathway C3 convertase) were found in a significantly higher proportion of children with the disorder versus children with hypocomplementemic C3 glomerulopathy (31 of 34 [91%] versus 4 of 28 [14%], respectively). In acute postinfectious GN, anti-factor B autoantibodies were transient and correlated with plasma C3 and soluble C5b-9 levels. We demonstrated that anti-factor B antibodies enhance alternative pathway convertase activity in vitro, confirming their pathogenic effect. We also identified crucial antibody binding sites on factor B, including one correlated to disease severity.
Conclusions: These findings elucidate the pathophysiologic mechanisms underlying acute postinfectious GN by identifying anti-factor B autoantibodies as contributing factors in alternative complement pathway activation. At onset of a nephritic syndrome with low C3 level, screening for anti-factor B antibodies might help guide indications for kidney biopsy to avoid misdiagnosed chronic glomerulopathy, such as C3 glomerulopathy, and to help determine therapy.
Keywords: complement; glomerulonephritis; pediatrics.
Copyright © 2020 by the American Society of Nephrology.
Figures


Comment in
-
Bacterial infection linked to anti-factor B antibodies.Nat Rev Nephrol. 2020 May;16(5):252. doi: 10.1038/s41581-020-0263-z. Nat Rev Nephrol. 2020. PMID: 32099192 No abstract available.
-
Challenges in Understanding Acute Postinfectious Glomerulonephritis: Are Anti-Factor B Autoantibodies the Answer?J Am Soc Nephrol. 2020 Apr;31(4):670-672. doi: 10.1681/ASN.2020020168. Epub 2020 Mar 6. J Am Soc Nephrol. 2020. PMID: 32144171 Free PMC article. No abstract available.
References
-
- Carapetis JR, Steer AC, Mulholland EK, Weber M: The global burden of group A streptococcal diseases. Lancet Infect Dis 5: 685–694, 2005. - PubMed
-
- Kambham N: Postinfectious glomerulonephritis. Adv Anat Pathol 19: 338–347, 2012. - PubMed
-
- Rodriguez-Iturbe B, Haas M: Post-streptococcal glomerulonephritis. In: Streptococcus pyogenes : Basic Biology to Clinical Manifestations, edited by Ferretti JJ, Stevens DL, Fischetti VA, Oklahoma City, OK, University of Oklahoma Health Sciences Center, 2016. Available at: http://www.ncbi.nlm.nih.gov/books/NBK333429/ - PubMed
-
- Yoshizawa N, Yamakami K, Fujino M, Oda T, Tamura K, Matsumoto K, et al. .: Nephritis-associated plasmin receptor and acute poststreptococcal glomerulonephritis: Characterization of the antigen and associated immune response. J Am Soc Nephrol 15: 1785–1793, 2004. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

