De novo strategy with engineering anti-Kasha/Kasha fluorophores enables reliable ratiometric quantification of biomolecules
- PMID: 32034152
- PMCID: PMC7005775
- DOI: 10.1038/s41467-020-14615-3
De novo strategy with engineering anti-Kasha/Kasha fluorophores enables reliable ratiometric quantification of biomolecules
Abstract
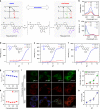
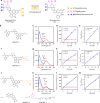
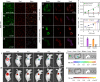
Fluorescence-based technologies have revolutionized in vivo monitoring of biomolecules. However, significant technical hurdles in both probe chemistry and complex cellular environments have limited the accuracy of quantifying these biomolecules. Herein, we report a generalizable engineering strategy for dual-emission anti-Kasha-active fluorophores, which combine an integrated fluorescein with chromene (IFC) building block with donor-π-acceptor structural modification. These fluorophores exhibit an invariant near-infrared Kasha emission from the S1 state, while their anti-Kasha emission from the S2 state at around 520 nm can be finely regulated via a spirolactone open/closed switch. We introduce bio-recognition moieties to IFC structures, and demonstrate ratiometric quantification of cysteine and glutathione in living cells and animals, using the ratio (S2/S1) with the S1 emission as a reliable internal reference signal. This de novo strategy of tuning anti-Kasha-active properties expands the in vivo ratiometric quantification toolbox for highly accurate analysis in both basic life science research and clinical applications.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

