Inflammation and matrix metalloproteinase 9 (Mmp-9) regulate photoreceptor regeneration in adult zebrafish
- PMID: 32034934
- PMCID: PMC7317489
- DOI: 10.1002/glia.23792
Inflammation and matrix metalloproteinase 9 (Mmp-9) regulate photoreceptor regeneration in adult zebrafish
Abstract
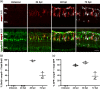
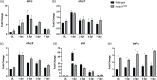
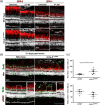
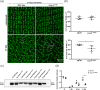
Brain injury activates complex inflammatory signals in dying neurons, surviving neurons, and glia. Here, we establish that inflammation regulates the regeneration of photoreceptors in the zebrafish retina and determine the cellular expression and function of the inflammatory protease, matrix metalloproteinase 9 (Mmp-9), during this regenerative neurogenesis. Following photoreceptor ablation, anti-inflammatory treatment suppresses the number of injury-induced progenitors and regenerated photoreceptors. Upon photoreceptor injury, mmp-9 is induced in Müller glia and Müller glia-derived photoreceptor progenitors. Deleting mmp-9 results in over production of injury-induced progenitors and regenerated photoreceptors, but over time the absence of Mmp-9 compromises the survival of the regenerated cones. At all time-points studied, the levels of tnf-α are significantly elevated in mutant retinas. Anti-inflammatory treatment in mutants rescues the defects in cone survival. These data provide a link between injury-induced inflammation in the vertebrate CNS, Mmp-9 function during neuronal regeneration and the requirement of Mmp-9 for the survival of regenerated cones.
Keywords: Müller glia; cytokines; immunosuppression; microglia; proliferation; stem cell.
© 2020 The Authors. Glia published by Wiley Periodicals, Inc.
Conflict of interest statement
The authors declare no potential conflict of interest.
Figures







References
-
- Ando, K. , Shibata, E. , Hans, S. , Brand, M. , & Kawakami, A. (2017). Osteoblast production by reserved progenitor cells in zebrafish bone regeneration and maintenance. Developmental Cell, 43, 643–650.e3. - PubMed
-
- Bernardos, R. L. , & Raymond, P. A. (2006). GFAP transgenic zebrafish. Gene Expression Patterns, 6, 1007–1013. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

