Fulvestrant plus capivasertib versus placebo after relapse or progression on an aromatase inhibitor in metastatic, oestrogen receptor-positive breast cancer (FAKTION): a multicentre, randomised, controlled, phase 2 trial
- PMID: 32035020
- PMCID: PMC7052734
- DOI: 10.1016/S1470-2045(19)30817-4
Fulvestrant plus capivasertib versus placebo after relapse or progression on an aromatase inhibitor in metastatic, oestrogen receptor-positive breast cancer (FAKTION): a multicentre, randomised, controlled, phase 2 trial
Abstract
Background: Capivasertib (AZD5363) is a potent selective oral inhibitor of all three isoforms of the serine/threonine kinase AKT. The FAKTION trial investigated whether the addition of capivasertib to fulvestrant improved progression-free survival in patients with aromatase inhibitor-resistant advanced breast cancer.
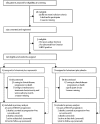
Methods: In this randomised, double-blind, placebo-controlled, phase 2 trial, postmenopausal women aged at least 18 years with an Eastern Cooperative Oncology Group performance status of 0-2 and oestrogen receptor-positive, HER2-negative, metastatic or locally advanced inoperable breast cancer who had relapsed or progressed on an aromatase inhibitor were recruited from 19 hospitals in the UK. Enrolled participants were randomly assigned (1:1) to receive intramuscular fulvestrant 500 mg (day 1) every 28 days (plus a loading dose on day 15 of cycle 1) with either capivasertib 400 mg or matching placebo, orally twice daily on an intermittent weekly schedule of 4 days on and 3 days off (starting on cycle 1 day 15) until disease progression, unacceptable toxicity, loss to follow-up, or withdrawal of consent. Treatment allocation was done using an interactive web-response system using a minimisation method (with a 20% random element) and the following minimisation factors: measurable or non-measurable disease, primary or secondary aromatase inhibitor resistance, PIK3CA status, and PTEN status. The primary endpoint was progression-free survival with a one-sided alpha of 0·20. Analyses were done by intention to treat. Recruitment is complete, and the trial is in follow-up. This trial is registered with ClinicalTrials.gov, number NCT01992952.
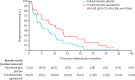
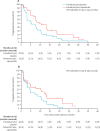
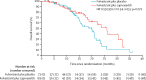
Findings: Between March 16, 2015, and March 6, 2018, 183 patients were screened for eligibility, of whom 140 (76%) were eligible and were randomly assigned to receive fulvestrant plus capivasertib (n=69) or fulvestrant plus placebo (n=71). Median follow-up for progression-free survival was 4·9 months (IQR 1·6-11·6). At the time of primary analysis for progression-free survival (Jan 30, 2019), 112 progression-free survival events had occurred, 49 (71%) in 69 patients in the capivasertib group compared with 63 (89%) of 71 in the placebo group. Median progression-free survival was 10·3 months (95% CI 5·0-13·2) in the capivasertib group versus 4·8 months (3·1-7·7) in the placebo group, giving an unadjusted hazard ratio (HR) of 0·58 (95% CI 0·39-0·84) in favour of the capivasertib group (two-sided p=0·0044; one-sided log rank test p=0·0018). The most common grade 3-4 adverse events were hypertension (22 [32%] of 69 patients in the capivasertib group vs 17 [24%] of 71 in the placebo group), diarrhoea (ten [14%] vs three [4%]), rash (14 [20%] vs 0), infection (four [6%] vs two [3%]), and fatigue (one [1%] vs three [4%]). Serious adverse reactions occurred only in the capivasertib group, and were acute kidney injury (two), diarrhoea (three), rash (two), hyperglycaemia (one), loss of consciousness (one), sepsis (one), and vomiting (one). One death, due to atypical pulmonary infection, was assessed as possibly related to capivasertib treatment. One further death in the capivasertib group had an unknown cause; all remaining deaths in both groups (19 in the capivasertib group and 31 in the placebo group) were disease related.
Interpretation: Progression-free survival was significantly longer in participants who received capivasertib than in those who received placebo. The combination of capivasertib and fulvestrant warrants further investigation in phase 3 trials.
Funding: AstraZeneca and Cancer Research UK.
Copyright © 2020 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Figures
Comment in
-
Capivasertib inhibits a key pathway in metastatic breast cancer.Lancet Oncol. 2020 Mar;21(3):318-319. doi: 10.1016/S1470-2045(19)30857-5. Epub 2020 Feb 5. Lancet Oncol. 2020. PMID: 32035019 No abstract available.
-
Fulvestrant plus capivasertib for metastatic breast cancer.Lancet Oncol. 2020 May;21(5):e232. doi: 10.1016/S1470-2045(20)30153-4. Lancet Oncol. 2020. PMID: 32359497 No abstract available.
-
Fulvestrant plus capivasertib for metastatic breast cancer.Lancet Oncol. 2020 May;21(5):e233. doi: 10.1016/S1470-2045(20)30228-X. Lancet Oncol. 2020. PMID: 32359498 No abstract available.
-
Fulvestrant plus capivasertib for metastatic breast cancer - Authors' reply.Lancet Oncol. 2020 May;21(5):e234. doi: 10.1016/S1470-2045(20)30237-0. Lancet Oncol. 2020. PMID: 32359499 No abstract available.
References
-
- deGraffenried LA, Friedrichs WE, Russell DH. Inhibition of mTOR activity restores tamoxifen response in breast cancer cells with aberrant Akt Activity. Clin Cancer Res. 2004;10:8059–8067. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

