RIG-I and TLR4 responses and adverse outcomes in pediatric influenza-related critical illness
- PMID: 32035159
- PMCID: PMC7323584
- DOI: 10.1016/j.jaci.2020.01.040
RIG-I and TLR4 responses and adverse outcomes in pediatric influenza-related critical illness
Abstract
Background: Decreased TNF-α production in whole blood after ex vivo LPS stimulation indicates suppression of the Toll-like receptor (TLR)4 pathway. This is associated with increased mortality in pediatric influenza critical illness. Whether antiviral immune signaling pathways are also suppressed in these patients is unclear.
Objectives: We sought to evaluate suppression of the TLR4 and the antiviral retinoic acid-inducible gene-I (RIG-I) pathways with clinical outcomes in children with severe influenza infection.
Methods: In this 24-center, prospective, observational cohort study of children with confirmed influenza infection, blood was collected within 72 hours of intensive care unit admission. Ex vivo whole blood stimulations were performed with matched controls using the viral ligand polyinosinic-polycytidylic acid-low-molecular-weight/LyoVec and LPS to evaluate IFN-α and TNF-α production capacities (RIG-I and TLR4 pathways, respectively).
Results: Suppression of either IFN-α or TNF-α production capacity was associated with longer duration of mechanical ventilation and hospitalization, and increased organ dysfunction. Children with suppression of both RIG-I and TLR4 pathways (n = 33 of 103 [32%]) were more likely to have prolonged (≥7 days) multiple-organ dysfunction syndrome (30.3% vs 8.6%; P = .004) or prolonged hypoxemic respiratory failure (39.4% vs 11.4%; P = .001) compared with those with single- or no pathway suppression.
Conclusions: Suppression of both RIG-I and TLR4 signaling pathways, essential for respective antiviral and antibacterial responses, is common in previously immunocompetent children with influenza-related critical illness and is associated with bacterial coinfection and adverse outcomes. Prospective testing of both pathways may aid in risk-stratification and in immune monitoring.
Keywords: IFN-α; LPS; Polyinosinic:polycytidylic acid; TNF-α; Toll-like receptor 4; critical care; influenza; pediatric; retinoic acid–inducible gene-I; suppression.
Copyright © 2020 American Academy of Allergy, Asthma & Immunology. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures

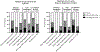
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

