Effect of alirocumab on individuals with type 2 diabetes, high triglycerides, and low high-density lipoprotein cholesterol
- PMID: 32035487
- PMCID: PMC7007683
- DOI: 10.1186/s12933-020-0991-1
Effect of alirocumab on individuals with type 2 diabetes, high triglycerides, and low high-density lipoprotein cholesterol
Abstract
Background: Mixed dyslipidemia [elevated non-high-density lipoprotein cholesterol (non-HDL-C) and triglycerides (TGs), and decreased HDL-C] is common in type 2 diabetes mellitus (T2DM) and is associated with increased cardiovascular risk. Non-HDL-C and apolipoprotein B (ApoB) are the preferred therapeutic targets for mixed dyslipidemia. Alirocumab is a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 (PCSK9) that effectively reduces low-density lipoprotein cholesterol (LDL-C), non-HDL-C, ApoB, and lipoprotein(a) (Lp[a]), and is well-tolerated in individuals with T2DM.
Methods: The previously reported open-label ODYSSEY DM-DYSLIPIDEMIA trial data demonstrated the effects of alirocumab on individuals with non-HDL-C ≥ 100 mg/dL and TGs ≥ 150 and < 500 mg/dL receiving stable maximally tolerated statin (n = 413). This post hoc subgroup analysis of the primary trial investigated the effects of alirocumab [75 mg every 2 weeks (Q2W) with possible increase to 150 mg Q2W at Week 12] versus usual care [ezetimibe, fenofibrate, or no additional lipid-lowering therapy (LLT)] on non-HDL-C and other lipids in individuals with T2DM and baseline TGs ≥ 200 mg/dL and HDL-C < 40 mg/dL (men) or < 50 mg/dL (women).
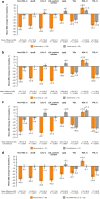
Results: Alirocumab significantly reduced non-HDL-C [LS mean difference (standard error (SE)), - 35.0% (3.9)], ApoB [LS mean difference (SE), - 34.7% (3.6)], LDL-C [LS mean difference (SE), - 47.3% (5.2)], LDL particle number [LS mean difference (SE), - 40.8% (4.1)], and Lp(a) [LS mean difference (SE), - 29.9% (5.4)] versus usual care from baseline to Week 24 (all P < 0.0001). Results were similar for alirocumab versus usual care. TG reductions were similar between alirocumab and usual care (no significant difference), but greater with fenofibrate versus alirocumab (P = 0.3371). Overall, alirocumab significantly increased HDL-C versus usual care [LS mean difference (SE), 7.9% (3.6); P < 0.05], although differences with alirocumab versus ezetimibe or fenofibrate were non-significant. Most individuals receiving alirocumab achieved ApoB < 80 mg/dL (67.9%) and non-HDL-C < 100 mg/dL (60.9%). Adverse event frequency was similar between alirocumab (67.2%) and usual care (70.7%). Additionally, no clinically relevant effect of alirocumab on change in glycemic parameters or use of antihyperglycemic agents was observed.
Conclusions: Alirocumab is an effective therapeutic option for individuals with T2DM, TGs ≥ 200 mg/dL, and HDL-C < 40 mg/dL (men) or < 50 mg/dL (women). Atherogenic lipid (ApoB and non-HDL) reductions were greater with alirocumab than ezetimibe, fenofibrate, or no LLT. Consistent with previous studies, alirocumab was generally well tolerated. Trial registration Clinicaltrials.gov, NCT02642159. Registered December 24, 2015, https://clinicaltrials.gov/ct2/show/NCT02642159.
Keywords: Alirocumab; DM-DYSLIPIDEMIA; Diabetes mellitus; HDL-C; Non-HDL-C; ODYSSEY; PCSK9; Triglycerides; Type 2 diabetes; Usual care.
Conflict of interest statement
HMC has received speaker’s bureau and consultant/advisory board fees from Sanofi Aventis, Regeneron Pharmaceuticals, Inc., Novartis Pharmaceuticals, Novo-Nordisk, and Eli Lilly; has received non-binding research support from Pfizer Inc., AstraZeneca LP, and Novo-Nordisk; and is a shareholder of Roche Pharmaceuticals and Bayer. LAL has received grants and personal fees from Amgen, AstraZeneca, Eli Lilly and Company, Esperion, HLS, Merck, Regeneron Pharmaceuticals, Inc., and Sanofi; and grants from Kowa and the Medicines Company. DM-W has received consultant fees/honoraria from Amgen Inc., AstraZeneca, Boehringer Ingelheim Pharmaceuticals, Inc., Merck & Co., Inc., Novartis Corporation, Novo Nordisk Inc., and Sanofi-Aventis; and participated in speaker’s bureau for Amgen Inc., AstraZeneca, Boehringer Ingelheim Pharmaceuticals, Inc., Eli Lilly and Company, Merck & Co., Inc., Novartis Corporation, Novo Nordisk Inc., and Sanofi-Aventis. BC has received research funding and personal fees from Sanofi and Regeneron Pharmaceuticals, Inc.; research funding from Amgen and Pfizer; and honoraria from Amgen, Akcea, AstraZeneca, Pierre Fabre, Genfit, Gilead, Eli Lilly and Company, MSD (Merck & Co.), Novo Nordisk, Sanofi, and Servier. KKR has received research grants from Pfizer Inc., Amgen, Sanofi, Regeneron Pharmaceuticals, Inc., and MSD; honoraria from Dr Reddy’s Laboratories, Zuellig Pharma, Sanofi, Amgen, Boehringer Ingelheim, Novo Nordisk, and Pfizer Inc.; and consultant/advisory board fees from Medco, AstraZeneca, Resverlogix, Kowa, Abbvie, Sanofi, Amgen, Boehringer Ingelheim, Esperion, Akcea, and Regeneron Pharmaceuticals, Inc. FJT has received speaker’s bureau and consultant/advisory board fees from AstraZeneca, Amgen, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly and Company, GlaxoSmithKline, Janssen Pharmaceuticals, MSD (Merck & Co.), Novartis Pharmaceuticals Co., Novo Nordisk, and Sanofi-Aventis. CD and AL are employees of and stockholders in Sanofi. MKI and RS are employees of and stockholders in Regeneron Pharmaceuticals, Inc. SDP has received research funding from AstraZeneca, Boehringer Ingelheim, Novartis Pharmaceuticals Co., and MSD (Merck & Co.); and has been a consultant for or received honoraria from AstraZeneca, Boehringer Ingelheim, Eli Lilly and Company, GlaxoSmithKline, Janssen Pharmaceuticals, Laboratoires Servier, MSD (Merck & Co.), Novartis Pharmaceuticals Co., Novo Nordisk, Sanofi-Aventis, and Takeda Pharmaceuticals.
Figures


References
-
- Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:3168–3209. doi: 10.1016/j.jacc.2018.11.002. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

