Surgical explantation of transcatheter aortic bioprostheses: Results and clinical implications
- PMID: 32037245
- PMCID: PMC7388726
- DOI: 10.1016/j.jtcvs.2019.11.139
Surgical explantation of transcatheter aortic bioprostheses: Results and clinical implications
Abstract
Objective: Despite the rapid adoption of transcatheter aortic valve replacement (TAVR) and worldwide interest in its implantation, TAVR valve explantation has not been well described.
Methods: We retrospectively reviewed 1442 consecutive patients who underwent a TAVR procedure between 2011 and 2019, in which TAVR explantation was performed in 15 patients (1.0%). In addition, 2 patients from outside institutions also underwent TAVR explantation at our institution. We reviewed the clinical details of these 17 patients.
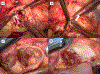
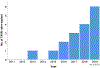
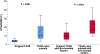
Results: The frequency of TAVR explant increased over time from 0 to 1 during the period from 2011 to 2015 to 6 in 2019. The mean age was 73.0 ± 9.3 years. The majority of patients (88.2%) were in New York Heart Association functional class IV heart failure. The Society of Thoracic Surgeons Predicted Risk of Mortality score was significantly higher at the time of explantation than at the time of the original TAVR (3.5% vs 9.9%; P < .001). The indication for explantation included structural valve degeneration (23.5%), severe paravalvular leak (41.2%), TAVR procedure-related complications (23.5%), endocarditis (5.9%), and bridge-to-definitive surgery (5.9%). Neoendothelialization of the TAVR valve into the aortic wall requiring intense aortic endarterectomy was noted in all 5 of the TAVR valves older than 1 year, in which 2 (40%) required unplanned aortic root repair. There were 2 (11.8%) in-hospital mortalities.
Conclusions: Surgical TAVR valve explant is increasing and may become common in the near future. The clinical effects of explanting chronically implanted valves with the potential need for aortic repair is not negligible. These data should be used to more appropriately select TAVR candidates as TAVR practices expand into younger and lower risk patients.
Keywords: aortic endarterectomy; aortic root replacement; structural valve degeneration; surgical aortic valve replacement; transcatheter aortic valve replacement.
Copyright © 2020 The American Association for Thoracic Surgery. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures


Comment in
-
Commentary: Good information is the best medicine.J Thorac Cardiovasc Surg. 2021 Aug;162(2):549-550. doi: 10.1016/j.jtcvs.2019.12.036. Epub 2020 Jan 3. J Thorac Cardiovasc Surg. 2021. PMID: 32014327 No abstract available.
-
Commentary: Aortic stenosis in young patients-planning a lifetime of aortic valve disease.J Thorac Cardiovasc Surg. 2021 Aug;162(2):548-549. doi: 10.1016/j.jtcvs.2019.12.095. Epub 2020 Jan 11. J Thorac Cardiovasc Surg. 2021. PMID: 32063356 No abstract available.
References
-
- Barbanti M, Webb JG, Tamburino C, Van Mieghem NM, Makkar RR, Piazza N et al. Outcomes of Redo Transcatheter Aortic Valve Replacement for the Treatment of Postprocedural and Late Occurrence of Paravalvular Regurgitation and Transcatheter Valve Failure. Circ Cardiovasc Interv. 2016;9. - PubMed
-
- Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597–607. - PubMed
-
- Popma JJ, Adams DH, Reardon MJ, Yakubov SJ, Kleiman NS, Heimansohn D, et al. Transcatheter aortic valve replacement using a self-expanding bioprosthesis in patients with severe aortic stenosis at extreme risk for surgery. J Am Coll Cardiol. 2014;63:1972–81. - PubMed
-
- Mangi AA, Ramchandani M, Reardon M Surgical Removal and Replacement of Chronically Implanted Transcatheter Aortic Prostheses: How I Teach It. Ann Thorac Surg. 2018;105:12–14. - PubMed
-
- Kapadia SR, Leon MB, Makkar RR, Tuzcu EM, Svensson LG, Kodali S, et al. 5-year outcomes of transcatheter aortic valve replacement compared with standard treatment for patients with inoperable aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet. 2015;385:2485–91. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

